2,5-二溴-3-甲基噻吩 | 13191-36-1
中文名称
2,5-二溴-3-甲基噻吩
中文别名
3-甲基-2,5-二溴噻吩
英文名称
2,5-dibromo-3-methylthiophene
英文别名
2,5-dibromo-3-methylthiophen;2,5-Dibrom-3-methylthiophen
CAS
13191-36-1
化学式
C5H4Br2S
mdl
MFCD00015470
分子量
255.961
InChiKey
IHFXZROPBCBLLG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:130-132 °C
-
沸点:226-230°C
-
密度:1.974 g/mL at 25 °C
-
闪点:226-230°C
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物和光接触。
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:28.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36
-
危险类别码:R36/38
-
WGK Germany:3
-
危险品运输编号:UN 2810 6.1 / PGIII
-
海关编码:2934999090
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302,H315,H318
-
储存条件:常温密闭,阴凉通风干燥
SDS
2,5-Dibromo-3-methylthiophene Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,5-Dibromo-3-methylthiophene
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,5-Dibromo-3-methylthiophene
Percent: >98.0%(GC)
CAS Number: 13191-36-1
Chemical Formula: C5H4Br2S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2,5-Dibromo-3-methylthiophene
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a refrigerator.
Store away from incompatible materials such as oxidizing agents.
Heat-sensitive, Light-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
2,5-Dibromo-3-methylthiophene
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Pale yellow - Yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
108°C/2.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.99
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
4.14
Log Pow:
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides, Hydrogen bromide
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: 4.14
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
2,5-Dibromo-3-methylthiophene
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,5-Dibromo-3-methylthiophene
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,5-Dibromo-3-methylthiophene
Percent: >98.0%(GC)
CAS Number: 13191-36-1
Chemical Formula: C5H4Br2S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2,5-Dibromo-3-methylthiophene
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a refrigerator.
Store away from incompatible materials such as oxidizing agents.
Heat-sensitive, Light-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
2,5-Dibromo-3-methylthiophene
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Pale yellow - Yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
108°C/2.3kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.99
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
4.14
Log Pow:
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides, Hydrogen bromide
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: 4.14
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
2,5-Dibromo-3-methylthiophene
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
2,5-二溴-3-甲基噻吩是一种杂环衍生物,主要用于制备医药中间体。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴-3-甲基噻吩 2-bromo-3-methylthiophene 14282-76-9 C5H5BrS 177.065 —— (2,5-dibromothiophen-3-yl)methanol 161490-95-5 C5H4Br2OS 271.96 2,5-二溴-3-(溴甲基)噻吩 2,5-dibromo-3-bromomethylthiophene 13191-37-2 C5H3Br3S 334.857 2,5-二溴噻吩-3-甲醛 2,5-dibromo thiophene-3-carboxaldehyde 1193-69-7 C5H2Br2OS 269.944 2,5-二溴-3-(二溴甲基)噻吩 2,5-dibromo-3-dibromomethylthiophen 57846-06-7 C5H2Br4S 413.753
反应信息
-
作为反应物:描述:2,5-二溴-3-甲基噻吩 在 (2,2'-bipyridine)nickel(II) dibromide tetrabutylammonium tetrafluoroborate 、 zinc dibromide 、 碘 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 生成 2-bromo-5-iodo-3-methylthiophene参考文献:名称:New and efficient access to 3-substituted 2,5-dibromothiophenes. Consecutive nickel-catalyzed electrochemical conversion to thienylzinc species摘要:我们已经通过电化学方法和一种原创的溴化程序实现了3取代噻吩锌试剂的逐步合成。对于几个化合物,3-溴噻吩是起始底物,它根据最近开发的电化学程序进行了功能化。在锌盐存在下,对得到的3取代2,5-二溴噻吩进行镍催化的电化学还原,使得单噻吩锌物种以良好的产率形成。关于该反应的选择性,我们在电化学合成有规聚噻吩的背景下进行了讨论。DOI:10.1039/b006748m
-
作为产物:描述:参考文献:名称:Side-chain engineering of benzodithiophene–thiophene copolymers with conjugated side chains containing the electron-withdrawing ethylrhodanine group摘要:设计并合成了四种含有具有电子吸引性的乙基罗丹宁受体单元的共轭侧链的苯二噻吩-噻吩共聚物。基于这四种聚合物的光伏电池展示出最高的光电转换效率为4.25%。DOI:10.1039/c5ta02360b
文献信息
-
Optical Activity of Heteroaromatic Conjugated Polymer Films Prepared by Asymmetric Electrochemical Polymerization in Cholesteric Liquid Crystals: Structural Function for Chiral Induction作者:Kohsuke Kawabata、Masaki Takeguchi、Hiromasa GotoDOI:10.1021/ma400302j日期:2013.3.26We electrochemically polymerized various achiral heteroaromatic monomers in left-handed helical cholesteric liquid crystal (CLC) media. Circular dichroism (CD) spectroscopy revealed that most of the resulting conjugated polymer films exhibited both the first negative and second positive Cotton effects near their absorption maxima. This indicates left-handed helical aggregation of the conjugated main我们在左旋螺旋胆甾型液晶(CLC)介质中电化学聚合了各种非手性杂芳族单体。圆二色性(CD)光谱显示,大多数所得共轭聚合物薄膜在其吸收最大值附近均表现出第一负棉花效应和第二正棉花效应。这表明共轭主链的左旋螺旋聚集,这与CLC的左旋螺旋顺序一致。该结果表明,在电沉积期间,左旋螺旋CLC环境引起聚合物的左旋螺旋聚集。然而,聚合物的CD强度取决于母体单体的结构。
-
作为丙型肝炎抑制剂的螺环化合物及其在药 物中的应用
-
Poly[3-(5-octyl-thienylene-vinyl)-thiophene]: A side-chain conjugated polymer with very broad absorption band作者:Jianhui Hou、Chunhe Yang、Chang He、Yongfang LiDOI:10.1039/b516576h日期:——A novel polythiophene derivative, poly[3-(5-octyl-thienylene-vinyl)-thiophene] (POTVT) with conjugated thienylene vinyl side-chain, was synthesized, and the POTVT film shows a very broad absorption band-width covering from 300 nm to 700 nm after thermal annealing at 130 °C for 10 min.
-
Novel dye sensitizers of main chain polymeric metal complexes based on complexes of 2-(2′-pyridyl)benzimidazole derivative with Zn(II), Co(II): synthesis, characterization, and photovoltaic performance for dye-sensitized solar cells作者:Dahai Peng、Wei Zhang、Guipeng Tang、Jun Zhou、Jiaomei Hu、Qiufang Xie、Chaofan ZhongDOI:10.1007/s13738-014-0539-y日期:2015.3AbstractIn this paper, four novel D–π–A polymeric metal complex as dye sensitizers for dye-sensitized solar cell, which have Poly(p-phenylenevinylene) or carbazole derivative used as an electron donor, thiophene derivatives used as π-bridge and 2-(2′-pyridyl)benzimidazole derivative metal (Zn, Co) complex used as an electron acceptor (A) were synthesized and characterized. They have been determined摘要在本文中,四种新型D–π–A聚合金属配合物作为染料敏化太阳能电池的染料敏化剂,它们具有Poly(p合成并表征了用作电子供体的-亚苯基亚乙烯基)或咔唑衍生物,用作π-桥的噻吩衍生物和用作电子受体(A)的2-(2'-吡啶基)苯并咪唑衍生物金属(Zn,Co)配合物。已通过FT-IR,TGA,DSC,GPC,元素分析,UV-vis吸收光谱,光致发光光谱,循环伏安法,J-V曲线和IPCE图确定并研究了它们。结果表明,四种新型聚合物金属配合物均表现出良好的光伏性能和热稳定性。由P1,P2,P3和P4制成的DSSC表现出良好的器件性能,功率转换效率分别高达2.15%,2.27%,2.30%和2.41%(在模拟空气质量1.5 G照射下),表明聚合物金属络合物有望在DSSC的发展。 图形概要 。
-
Highly Regioselective Preparation of Heteroaryl-Magnesium Reagents by Using a Br/Mg Exchange作者:Christoph Sämann、Benjamin Haag、Paul KnochelDOI:10.1002/chem.201202230日期:2012.12.7Disubstituted thienyl‐, furyl‐ and pyridylmagnesium derivatives are regioselectively prepared from a Br/Mg exchange of the corresponding dibromo compounds by using either iPrMgCl⋅LiCl or hindered arylmagnesium reagents, such as isitylmagnesium bromide⋅lithium chloride (isityl=2,4,6‐triisopropyl‐phenyl) complexed with a diamine ligand, in difficult cases. The selective functionalisations of these heterocyclic
表征谱图
-
氢谱1HNMR
-
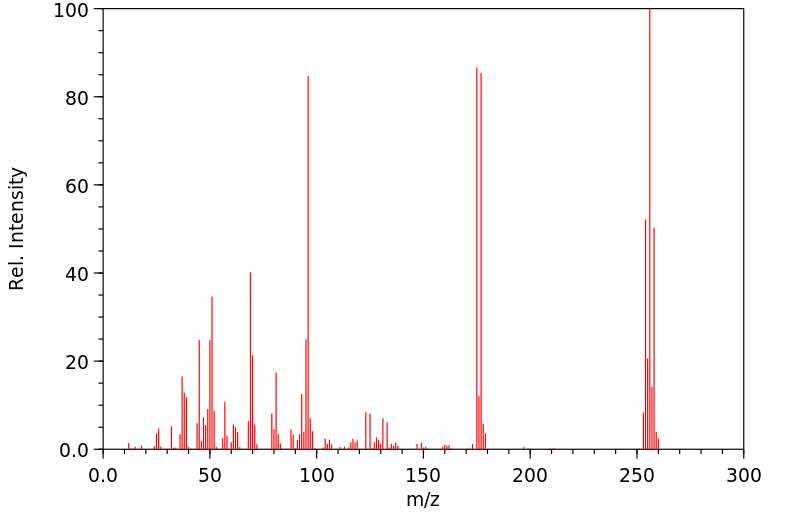
质谱MS
-
碳谱13CNMR
-
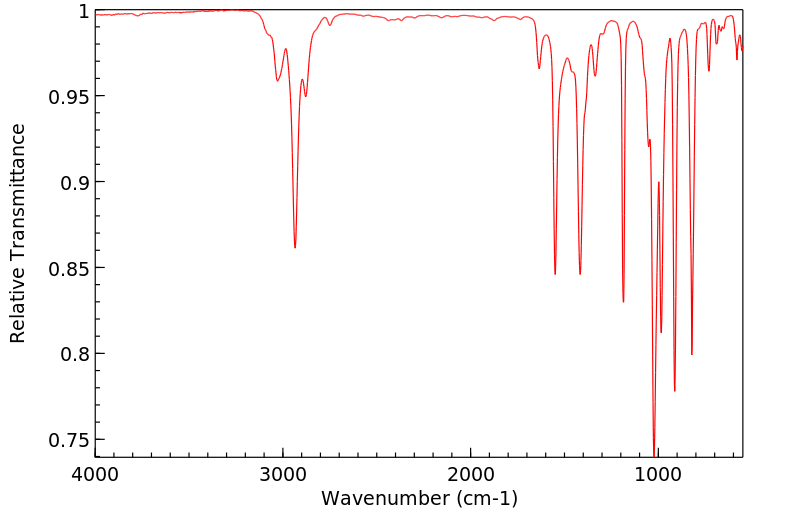
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯