灭螨猛 | 2439-01-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172°
-
沸点:476.6±55.0 °C(Predicted)
-
密度:1.4147 (rough estimate)
-
溶解度:氯仿(微溶)、甲醇(微溶、加热)
-
LogP:3.78 at 20℃
-
物理描述:Chinomethionat appears as yellow crystals. Non-corrosive. Used as a selective fungicide.
-
颜色/状态:Yellow crystals from benzene
-
气味:ODORLESS
-
蒸汽压力:0.026 mPa (2.0X10-7 mm Hg) at 20 °C
-
稳定性/保质期:
Relatively stable under normal conditions. Hydrolysed in alkaline media; DT50 (22 °C) 10 days (pH 4), 80 hr (pH 7), 225 min (pH 9).
-
分解:When heated to decomposition it emits very toxic fumes of /nitrogen and sulfur oxides/.
-
腐蚀性:Non-corrosive
-
碰撞截面:142.67 Ų [M+H]+
-
保留指数:2047;2081;2060;2068.4;2054.8;2038.2;2038.6;2053.5;2085.1
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:93.4
-
氢给体数:0
-
氢受体数:5
ADMET
安全信息
-
危险品标志:Xn,N
-
安全说明:S24,S37,S60,S61
-
危险类别码:R20/21/22
-
海关编码:2934999033
-
WGK Germany:3
-
危险品运输编号:UN3077 9/PG 3
制备方法与用途
简介 灭螨猛(chinomethionat),试验代号Bayer36205、Bayer SAS2074。商品名称为Morestan、Morestan VP,是由拜耳公司开发的杀菌杀螨剂。其化学名称为6-甲基-1,3-二硫戊环并[4,5-b]喹喔啉-2-酮或S,S-(6-甲基喹喔琳-2,3-二基)二硫代碳酸酯,即6-methyl-l,3-dithiolo[4,5-b]quinoxalin-2-one或S,S-(6-methylquinoxaline-2,3-diyl)dithiocarbonate。
理化性质 灭螨猛纯品为淡黄色结晶状固体,熔点170℃。其蒸气压在20℃时为0.026mPa。分配系数KowlgP=3.78(20℃),Henry常数为6.09×10-3Pa·m3/mol(计算值)。相对密度1.556(20℃);水中溶解度在20℃时为1g/L。在有机溶剂中的溶解度如下:甲苯25 g/L,二氯甲烷40 g/L,己烷1.8 g/L,异丙醇0.9 g/L,环己酮18 g/L,DMF10 g/L,石油醚4 g/L;热的甲苯和二氧六环也能溶解。常温下相对稳定,在碱性介质中分解;DTo(22℃):pH4时为10天,pH7时为80小时,pH9时为225分钟。
应用 灭螨猛用于控制水果(包括柑橘类)、观赏植物、葫芦、棉花、咖啡、茶、烟草、核桃、蔬菜和温室作物的白粉病和螨虫。对某些品种的苹果、梨、黑醋栗、玫瑰及观赏植物有药害。
作用机理 灭螨猛是一种选择性非内吸性触杀型杀菌剂,具有保护和铲除活性。
合成方法 灭螨猛以对甲苯胺为原料,经过一系列反应即可制得所需产品。具体步骤请参考相关文献或资料中的化学反应图示。
毒性 急性经口LD50(mg/kg):雄大鼠2541,雌大鼠1095;大鼠急性经皮LD50>5000 mg/kg。对兔皮肤有轻度刺激,对兔眼睛有强烈刺激;大鼠吸入LC50(4小时,mg/L空气):雄>4.7,雌>2.2。在饲料中的最大无作用剂量(NOEL, mg/kg饲料)为:大鼠40(两年),雄性小鼠270(两年),雌性小鼠<90(两年),狗25(一年)。ADI值为0.006 mg/kg。
类别 农药
毒性分级 中毒
急性毒性 口服- 大鼠 LD50: 1100 毫克/公斤
可燃性危险特性 燃烧时产生有毒氮氧化物和硫氧化物气体
储运特性 应存放在库房通风低温干燥处;与食品原料分开储存和运输
灭火剂 干粉、泡沫、砂土
反应信息
-
作为反应物:参考文献:名称:杀真菌剂chinomethionate作为光诱导DNA切割剂的新家族。摘要:在本报告中首次证明,在温和的辐照条件下,硫代硫氰酸酯能够引起有效的DNA裂解,这是一种杀真菌剂分子,可处理简单的1,3-二硫-2-酮基团作为其反应性官能团。DOI:10.1016/s0960-894x(03)00745-5
-
作为产物:描述:参考文献:名称:WO2022/202643摘要:公开号:
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
表征谱图
-
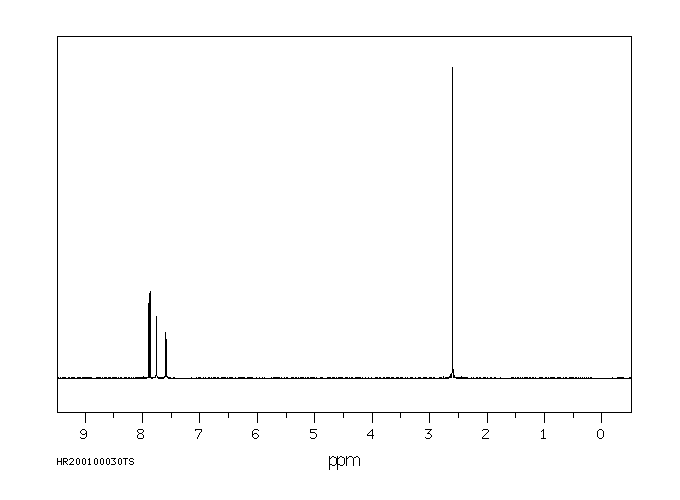
氢谱1HNMR
-
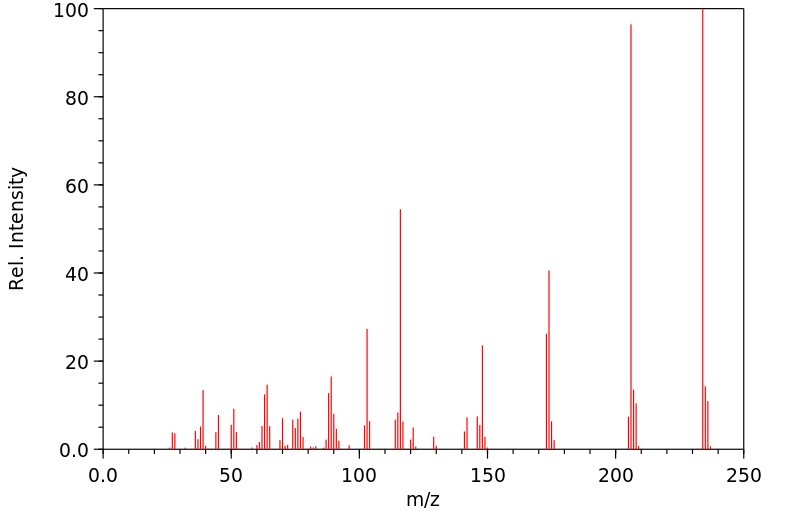
质谱MS
-
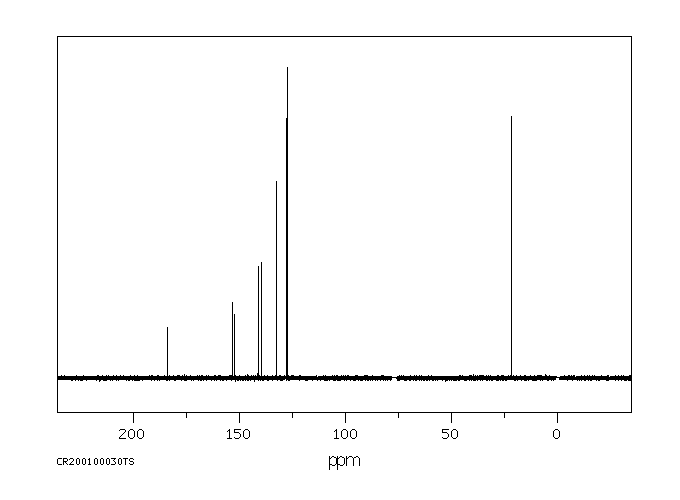
碳谱13CNMR
-
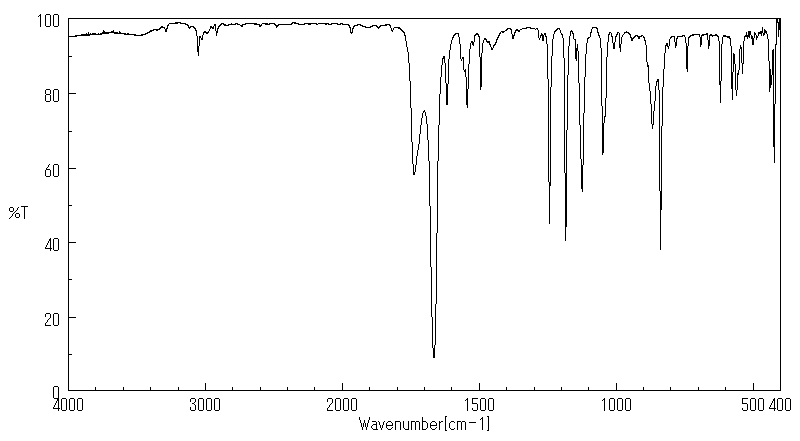
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息