烯丙基磷酸二甲酯 | 757-54-0
中文名称
烯丙基磷酸二甲酯
中文别名
烯丙基磷酸二甲酯,TECH
英文名称
allylphosphonic acid dimethyl ester
英文别名
dimethyl allylphosphonate;3-dimethoxyphosphorylprop-1-ene
CAS
757-54-0
化学式
C5H11O3P
mdl
MFCD00014951
分子量
150.114
InChiKey
ZOSQAGGCVFVCNO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:41-42 °C (0.5 mmHg)
-
密度:1.1378 g/cm3(Temp: 0 °C)
-
闪点:41-42°C/0.5mm
-
稳定性/保质期:
常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S24/25
-
储存条件:请将容器密封,并存放在干燥、阴凉处。
SDS
| Name: | Dimethyl Allylphosphonate 97% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 757-54-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 757-54-0 | Dimethyl Allylphosphonate | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use extinguishing media most appropriate for the surrounding fire.
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 757-54-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 41-42 deg C @ 0.5 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H11O3P
Molecular Weight: 150.049
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of phosphorus, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 757-54-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Dimethyl Allylphosphonate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 757-54-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 757-54-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 757-54-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 烯丙基膦酸 allylphosphonic acid 6833-67-6 C3H7O3P 122.061 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl hydrogen allylphosphonate —— C4H9O3P 136.087 —— tetramethyl 2-butene-1,4-diphosphonate 3858-16-0 C8H18O6P2 272.175 —— 1.4-Bis-dimethoxyphosphinyl-2-E-buten 56727-03-8 C8H18O6P2 272.175 烯丙基膦酸 allylphosphonic acid 6833-67-6 C3H7O3P 122.061
反应信息
-
作为反应物:描述:参考文献:名称:(E)-丁-2-烯基核苷膦酰胺酸酯的高度聚合合成和抗病毒活性。摘要:在超声辐射下,通过交叉复分解,用氮试剂指导制备了几种迄今未知的(E)-丁-2-烯基核苷磷酸氨基酰胺类似物(ANP)。通过X射线衍射正式鉴定出两种非对映异构体。这些化合物针对各种DNA和RNA病毒进行了评估。其中,磷酸氨基酰胺胸腺嘧啶类似物19成为对抗水痘带状疱疹病毒(VZV)的最佳前药,野生型和胸苷激酶缺陷型菌株的EC50值分别为0.33和0.39μM,选择性指数≥200μM。这一突破性方法为新的嘌呤和嘧啶(E)-丁-2-烯基磷酸氨基酰胺类似物铺平了道路。DOI:10.1016/j.ejmech.2018.01.086
-
作为产物:描述:参考文献:名称:Process for the production of (meth)allyl phosphonic acid dialkyl esters摘要:本发明涉及一种生产烯丙基或甲基烯丙基膦酸二烷基酯的方法,该方法通过在存在价态和/或一价镍化合物或存在价态、一价或二价钴化合物的情况下,将烯丙基氯化物或甲基烯丙基氯化物与三烷基膦酸酯在80℃至160℃的温度下反应而得到。公开号:US04017564A1
文献信息
-
New selectivities from old catalysts. Occlusion of Grubbs’ catalysts in PDMS to change their reactions作者:M. Brett Runge、Martin T. Mwangi、Ned B. BowdenDOI:10.1016/j.jorganchem.2006.09.022日期:2006.12dissolved in methylene chloride also react by olefin isomerization with occluded catalysts. Eleven examples of substrates that exhibit dual reactivity by undergoing olefin isomerization with occluded catalysts and olefin metathesis with catalysts dissolved in methylene chloride are reported. Most of these substrates have olefins with allylic phosphine oxides, carbonyls, or ethers. Control experiments demonstrate本文介绍了Grubbs第一代和第二代催化剂在聚二甲基硅氧烷(PDMS)的疏水性基质中被吸附时的新选择性。通过用二氯甲烷溶胀,然后在真空下除去溶剂,可将催化剂封闭在毫米大小的PDMS平板中。催化剂均相溶解在PDMS中,但仍保持催化活性。许多通过烯烃复分解反应与自由溶解在二氯甲烷中的Grubbs催化剂反应的底物也通过烯烃异构化与封闭的催化剂反应。报道了通过封闭的催化剂进行烯烃异构化和用溶解在二氯甲烷中的催化剂进行烯烃复分解而表现出双重反应性的底物的十一个实例。这些底物中的大多数具有带有烯丙基氧化膦,羰基或醚的烯烃。对照实验表明,通过催化剂从钌卡宾分解为拟议的氢化钌,溶剂中发生了异构化。通过将封闭的Grubbs的第一代催化剂在90%的MeOH / H中加热至100°C来扩展这项工作在各种烯烃的存在下将2 O转化为用于未官能化烯烃的Grubbs催化剂转化为异构化催化剂。这项工作表明,PDMS中有机
-
Radical Transfer Hydroamination with Aminated Cyclohexadienes Using Polarity Reversal Catalysis: Scope and Limitations作者:Joyram Guin、Christian Mück-Lichtenfeld、Stefan Grimme、Armido StuderDOI:10.1021/ja0692581日期:2007.4.1The synthesis of various new 1-aminated-2,5-cyclohexadienes is described. These reagents can be used in radical transfer hydroaminations of unactivated and electron-rich double bonds. With thiols as polarity reversal catalysts good yields are obtained. The radical hydroamination occurs with good to excellent anti-Markovnikov selectivity. Many functional groups such as alcohols, silyl ethers, phosphonates
-
A Practical Route to Cyclobutanols and Fluorocyclobutanes作者:Vincent L. Revil‐Baudard、Samir Z. ZardDOI:10.1002/hlca.202100106日期:2021.91-[(Ethoxycarbonothioyl)sulfanyl]cyclobutyl acetate (xanthate 7) was found to add to electronically unbiased alkenes and to certain heteroarenes. In the latter case, this corresponds to a variant of the Minisci reaction and allows the late-stage modification of biologically active substances. Saponification of the acetate furnishes the corresponding cyclobutanols, which, in the case of the nicotine
-
Expedient synthesis and biological evaluation of alkenyl acyclic nucleoside phosphonate prodrugs作者:Elisa Pileggi、Michaela Serpi、Graciela Andrei、Dominique Schols、Robert Snoeck、Fabrizio PertusatiDOI:10.1016/j.bmc.2018.05.034日期:2018.7The importance of phosphonoamidate prodrugs (ProTides) of acyclic nucleoside phosphonate (ANPs) is highlighted by the approval of Tenofovir Alafenamide Fumarate for the treatment of HIV and HBV infections. In the present paper we are reporting an expedient, one-pot, two-steps synthesis of allyl phosphonoamidates and diamidates that offers a time saving strategy when compared to literature methods.
-
The Shortest Strategy for Generating Phosphonate Prodrugs by Olefin Cross-Metathesis - Application to Acyclonucleoside Phosphonates作者:Ugo Pradère、Hervé Clavier、Vincent Roy、Steven P. Nolan、Luigi A. AgrofoglioDOI:10.1002/ejoc.201101111日期:2011.12A short synthetic route to phosphonate prodrugs by olefin cross-metathesis, which uses either (acyloxymethyl) or (hexadecyloxypropyl) allylphosphonate building blocks is described. A study of eight ruthenium catalysts including the Ru–indenylidene catalyst, which bears the N-heterocyclic carbene 1,3-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazol-2-ylidene, was undertaken. This method was applied to
表征谱图
-
氢谱1HNMR
-
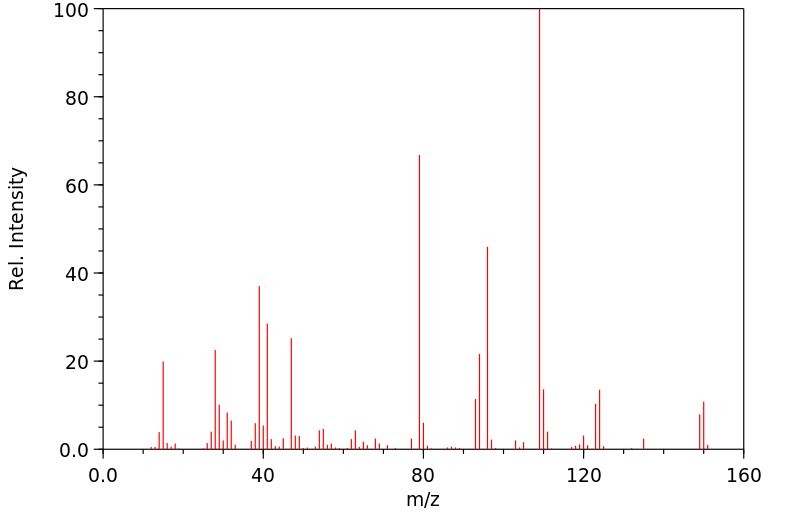
质谱MS
-
碳谱13CNMR
-
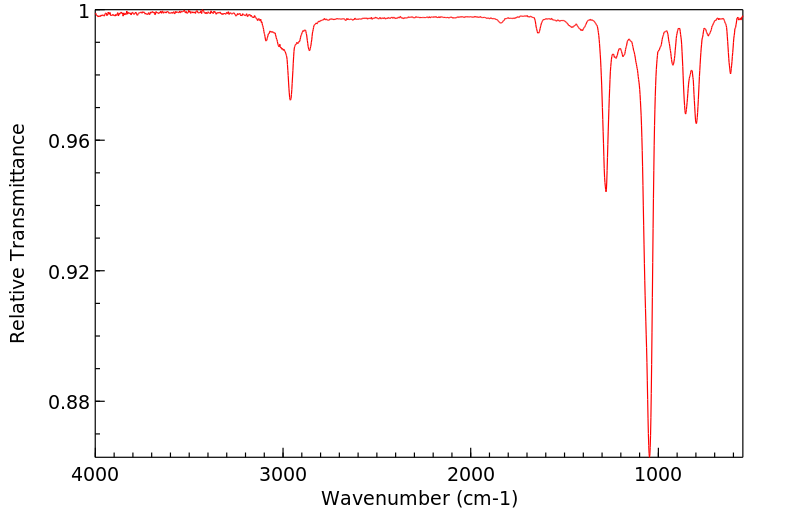
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1-氨基丁基)磷酸
顺丙烯基磷酸
除草剂BUMINAFOS
阿仑膦酸
阻燃剂 FRC-1
铵甲基膦酸盐
钠甲基乙酰基膦酸酯
钆1,5,9-三氮杂环十二烷-N,N',N''-三(亚甲基膦酸)
钆-1,4,7-三氮杂环壬烷-N,N',N''-三(亚甲基膦酸)
重氮甲基膦酸二乙酯
辛基膦酸二丁酯
辛基膦酸
辛基-膦酸二钾盐
辛-1-烯-2-基膦酸
试剂12-Azidododecylphosphonicacid
英卡膦酸
苯胺,4-乙烯基-2-(1-甲基乙基)-
苯甲基膦酸二甲酯
苯基膦酸二甲酯
苯基膦酸二仲丁酯
苯基膦酸二乙酯
苯基膦酸二乙酯
苯基磷酸二辛酯
苯基二异辛基亚磷酸酯
苯基(1H-1,2,4-三唑-1-基)甲基膦酸二乙酯
Tetrapotassium (((2-hydroxyethyl)imino)bis(methylene))bisphosphonate
苄基膦酸苄基乙酯
苄基亚甲基二膦酸
膦酸,[(2-乙基己基)亚氨基二(亚甲基)]二,triammonium盐(9CI)
膦酸叔丁酯乙酯
膦酸单十八烷基酯钾盐
膦酸二辛酯
膦酸二(二十一烷基)酯
膦酸,辛基-,单乙基酯
膦酸,甲基-,单(2-乙基己基)酯
膦酸,甲基-,二(苯基甲基)酯
膦酸,甲基-,2-甲氧基乙基1-甲基乙基酯
膦酸,丁基乙基酯
膦酸,[苯基[(苯基甲基)氨基]甲基]-,二甲基酯
膦酸,[[羟基(苯基甲基)氨基]苯基甲基]-,二(苯基甲基)酯
膦酸,[2-(环丙基氨基)-2-羰基乙基]-,二乙基酯
膦酸,[2-(二甲基亚肼基)丙基]-,二乙基酯,(E)-
膦酸,[1-甲基-2-(苯亚氨基)乙烯基]-,二乙基酯
膦酸,[1-(乙酰基氨基)-1-甲基乙基]-(9CI)
膦酸,[(环己基氨基)苯基甲基]-,二乙基酯
膦酸,[(二乙氧基硫膦基)(二甲氨基)甲基]-
膦酸,[(2S)-2-氨基-2-苯基乙基]-,二乙基酯
膦酸,[(1Z)-2-氨基-2-(2-噻嗯基)乙烯基]-,二乙基酯
膦酸,P-[(二乙胺基)羰基]-,二乙基酯
膦酸,(氨基二环丙基甲基)-