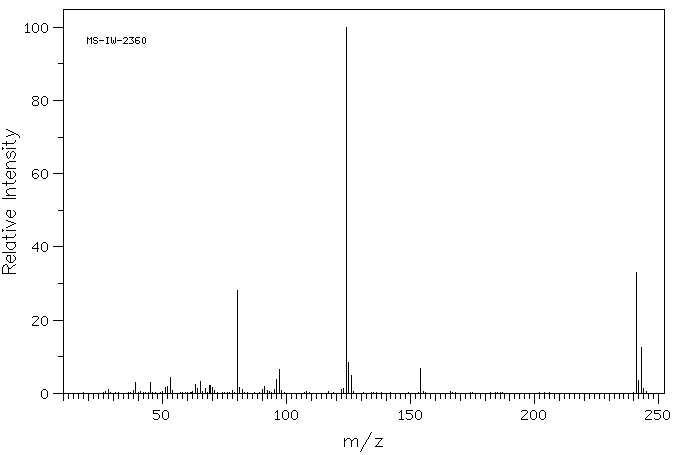
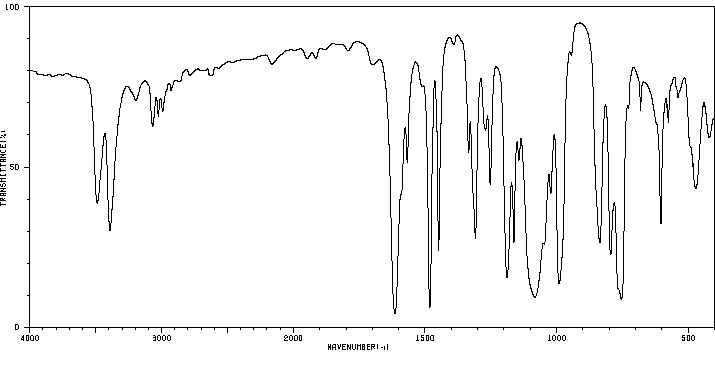
2-[(2-氯-1,1,2-三氟乙基)硫烷基]苯胺 | 81029-02-9
中文名称
2-[(2-氯-1,1,2-三氟乙基)硫烷基]苯胺
中文别名
2-[(2-氯-1,1,2-三氟乙基)硫代]苯胺;2-(2-氯-1,1,2-三氟乙硫基)苯胺
英文名称
2-[(2-Chloro-1,1,2-trifluoroethyl)thio]aniline
英文别名
2-(2-chloro-1,1,2-trifluoroethyl)sulfanylaniline
CAS
81029-02-9
化学式
C8H7ClF3NS
mdl
——
分子量
241.66
InChiKey
FCZIJASKFXRHTO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
反应信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:51.3
-
氢给体数:1
-
氢受体数:5
安全信息
-
海关编码:2930909090
文献信息
-
Inhibitors of C-FMS kinase申请人:Player R. Mark公开号:US20050113566A1公开(公告)日:2005-05-26The invention relates to compounds of Formula I: wherein A is phenyl, naphthyl or biphenyl, each of which may be optionally substituted with one or more of -C 1-6 alkyl, amino, aminoalkyl, hydroxyalkyl, alkoxyalkyl, sulfonamidoalkyl, guanidinoalkyl, heteroaryl, halogen, hydroxy, —CF 3 , alkoxy, aryl, aralkyl, heteroaralkyl, aryloxy, arylalkoxy, —OCF 3 , —OCO-alkyl, —CORA, —CN, —C(NH)NH 2 , —COOR a , —CONR a R b , —N(R a )COR b , —NO 2 , —SO 2 R a , —SO 3 R a or —SO 2 NR a R b ; or a 5- to 7-membered mono- or a 8- to 10-membered bicyclic heteroaromatic ring having from one to four heteroatoms selected from N, O or S, and may be optionally substituted with one or more of -C 1-6 alkyl, amino, aminoalkyl, hydroxyalkyl, alkoxyalkyl, sulfonamidoalkyl, guanidinoalkyl, heteroaryl, halogen, hydroxy, —CF 3 , alkoxy, aryl, aralkyl, heteroaralkyl, aryloxy, arylalkoxy, —OCF 3 , —OCO-alkyl, —COR a , —CN, —C(NH)NH 2 , —COOR a , —CONR a R b , —N(R a )COR b , —NO 2 , —SO 2 R a , —SO 3 R a or —SO 2 NR a R b ; R 1 is —H, aryl, —COR a , —COR a , —COOR a , —CONR a R b , —SO 2 R a or —SO 2 NR a R b ; X is —CO—, —C(═NH)—, —CS—, —CON(R a )—, —CS(NR a )—, —SO 2 — or —CR a R b —; Y is —S—, —SO—, —SO 2 —, —O— or direct link; R 2 is alkyl, cycloalkyl, heterocyclyl, aryl or heteroaryl, each of which may be optionally substituted with one or more halogens; and W is phenyl, naphthyl or biphenyl, each of which may be optionally substituted with one or more of C 1-4 alkyl, amino, aminoalkyl, hydroxyalkyl, alkoxyalkyl, halogen, hydroxy, —CF 3 , alkoxy, aryloxy, arylalkoxy, —OCF 3 , —COR a , —CN, —C(NH)NH 2 , —COOR a , —CONR a R b , —NHCOR a R b , —NHSO 2 R a , —NO 2 , —SOR a , —SO 3 R a or —SO 2 NR a R b ; or a 5- to 6-membered mono- or a 8- to 10-membered bicyclic heterocyclic or heteroaromatic ring having from one to four heteroatoms selected from N, O or S, and may be optionally substituted with -C 1-6 alkyl, amino, aminoalkyl, hydroxyalkyl, alkoxyalkyl, heteroaryl, halogen, hydroxy, —CF 3 , alkoxy, aryl, aralkyl, heteroaralkyl, aryloxy, arylalkoxy, —OCF 3 , —OCO-alkyl, —OCO-alkylamino, —OCO-alkylamido, COR a , —CN, —C(NH)NH 2 , —COOR a , —CONR a R b , —N(R a )COR b , —NO 2 , —SO 2 R a , —SO 3 R a or —SO 2 NR a R b ; as well as solvates, hydrates, tautomers or pharmaceutically acceptable salts thereof, that inhibit protein tyrosine kinases, especially c-fms kinase.该发明涉及Formula I的化合物: 其中A为苯基、萘基或联苯基,每种基团可以选择性地取代一个或多个以下基团之一:-C1-6烷基、氨基、氨基烷基、羟基烷基、烷氧基烷基、磺胺基烷基、胍基烷基、杂环芳基、卤素、羟基、—CF3、烷氧基、芳基、芳基烷基、杂环芳基、芳基氧基、芳基烷氧基、—O 、—OCO-烷基、—CORA、—CN、—C(NH)NH2、—COORA、—CONRARb、—N(RA)CORb、—NO2、—SO2RA、—SO3RA或—SO2NRARb;或者为含有1至4个异原子(N、O或S)的5至7成员的单环或8至10成员的双环杂芳环,可以选择性地取代一个或多个以下基团之一:-C1-6烷基、氨基、氨基烷基、羟基烷基、烷氧基烷基、磺胺基烷基、胍基烷基、杂环芳基、卤素、羟基、— 、烷氧基、芳基、芳基烷基、杂环芳基、芳基氧基、芳基烷氧基、—O 、—OCO-烷基、—CORA、—CN、—C(NH)NH2、—COORA、—CONRARb、—N(RA)CORb、— 、—SO2RA、—SO3RA或—SO2NRARb;R1为—H、芳基、—CORA、—CORA、—COORA、—CONRARb、—SO2RA或—SO2NRARb;X为—CO—、—C(═NH)—、—CS—、—CON(RA)—、—CS(NRA)—、—SO2—或—CRARb—;Y为—S—、—SO—、—SO2—、—O—或直链;R2为烷基、环烷基、杂环烷基、芳基或杂芳基,每种基团可以选择性地取代一个或多个卤素;W为苯基、萘基或联苯基,每种基团可以选择性地取代一个或多个以下基团之一:C1-4烷基、氨基、氨基烷基、羟基烷基、烷氧基烷基、卤素、羟基、— 、烷氧基、芳基氧基、芳基烷氧基、—O 、—CORA、—CN、—C(NH)NH2、—COORA、—CONRARb、—NHCORARb、—NHSO2RA、— 、—SORA、—SO3RA或—SO2NRARb;或者为含有1至4个异原子(N、O或S)的5至6成员的单环或8至10成员的双环杂环或杂芳环,可以选择性地取代一个或多个以下基团之一:-C1-6烷基、氨基、氨基烷基、羟基烷基、烷氧基烷基、杂环芳基、卤素、羟基、— 、烷氧基、芳基、芳基烷基、杂环芳基、芳基氧基、芳基烷氧基、—O 、—OCO-烷基、—OCO-烷基氨基、—OCO-烷基酰胺基、CORA、—CN、—C(NH)NH2、—COORA、—CONRARb、—N(RA)CORb、— 、—SO2RA、—SO3RA或—SO2NRARb;以及这些化合物的溶剂化合物、水合物、互变异构体或药学上可接受的盐,能抑制蛋白酪氨酸激酶,尤其是c-fms激酶。
-
C-fms kinase inhibitors申请人:Player R. Mark公开号:US20050004112A1公开(公告)日:2005-01-06The invention is directed to compounds of Formulae I, II and III: wherein A, R 1 , R 2 , R 3 , R 4 , X, Y and W are set forth in the specification, as well as solvates, hydrates, tautomers or pharmaceutically acceptable salts thereof, that inhibit protein tyrosine kinases, especially c-fms kinase.
-
c-fms kinase inhibitors申请人:Player R. Mark公开号:US20060258666A1公开(公告)日:2006-11-16The invention is directed to compounds of Formula II: wherein A, R 1 , R 2 , R 3 , R 4 , X, Y and W are set forth in the specification, as well as solvates, hydrates, tautomers or pharmaceutically acceptable salts thereof, that inhibit protein tyrosine kinases, especially c-fms kinase.
-
C-FMS KINASE INHIBITORS申请人:3-DIMENSIONAL PHARMACEUTICALS, INC.公开号:EP1631560A2公开(公告)日:2006-03-08
-
Negative curable dye-containing composition, color filter, and method of manufacturing the same申请人:Takakuwa Hideki公开号:US20070072096A1公开(公告)日:2007-03-29The invention provides a negative curable dye-containing composition including at least one compound selected from amine and pyridine compounds, a phthalocyanine dye soluble in an organic solvent, a photopolymerization initiator, and a radical polymerizable monomer, a color filter formed by using the same, and a manufacturing method of the color filter.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯