3-nitro-benzenesulfonic acid-[1]naphthylamide | 419559-79-8
中文名称
——
中文别名
——
英文名称
3-nitro-benzenesulfonic acid-[1]naphthylamide
英文别名
3-Nitro-N-(naphthyl-(1))-benzolsulfonamid-(1);3-Nitro-benzolsulfonsaeure-[1]naphthylamid;1-m-Nitrobenzolsulfonylaminonaphthalin;N-(1-Naphthyl)-3-nitrobenzenesulfonamide;N-naphthalen-1-yl-3-nitrobenzenesulfonamide
CAS
419559-79-8
化学式
C16H12N2O4S
mdl
——
分子量
328.348
InChiKey
WIFWFLHGNPQTSE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:23
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:100
-
氢给体数:1
-
氢受体数:5
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-amino-benzenesulfonic acid-[1]naphthylamide 132515-29-8 C16H14N2O2S 298.365
反应信息
-
作为反应物:描述:3-nitro-benzenesulfonic acid-[1]naphthylamide 在 N-甲基吗啉 、 tin(ll) chloride 、 氯甲酸异丁酯 作用下, 以 乙酸乙酯 、 N,N-二甲基甲酰胺 为溶剂, 反应 4.08h, 生成 Hexadecanoic acid {2-[3-(naphthalen-1-ylsulfamoyl)-phenylcarbamoyl]-ethyl}-amide参考文献:名称:Design, synthesis and early structure–activity relationship of farnesyltransferase inhibitors which mimic both the peptidic and the prenylic substrate摘要:Inhibition of the farnesylation of ras proteins has been identified as a promising target in tumor therapy. Only a few farnesyltransferase inhibitors are bisubstrate analogues displaying features of both substrates, the farnesylpyrophosphate and the C-terminal CAAX-tetrapeptide sequence of the res protein. These known bisubstrate analogues consist of an AAX-tripeptide and a farnesyl residue connected through various linkers. We have developed a class of novel compounds that mimic a bisubstrate inhibitor structure and that differ from the known ones by lacking peptidic or farnesylic substructures. Long chain fatty acids and aryl-substituted carboxylic acids were used as farnesyl surrogates. These structures were linked to isoleucine amide, benzoic acid amide, N-substituted aminobenzenesulfonamides and N-alpha-aryl-substituted methionine derivatives, respectively, which function as AA- or AAX-mimetics. (C) 2000 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0968-0896(00)00138-3
-
作为产物:描述:参考文献:名称:Design, synthesis and early structure–activity relationship of farnesyltransferase inhibitors which mimic both the peptidic and the prenylic substrate摘要:Inhibition of the farnesylation of ras proteins has been identified as a promising target in tumor therapy. Only a few farnesyltransferase inhibitors are bisubstrate analogues displaying features of both substrates, the farnesylpyrophosphate and the C-terminal CAAX-tetrapeptide sequence of the res protein. These known bisubstrate analogues consist of an AAX-tripeptide and a farnesyl residue connected through various linkers. We have developed a class of novel compounds that mimic a bisubstrate inhibitor structure and that differ from the known ones by lacking peptidic or farnesylic substructures. Long chain fatty acids and aryl-substituted carboxylic acids were used as farnesyl surrogates. These structures were linked to isoleucine amide, benzoic acid amide, N-substituted aminobenzenesulfonamides and N-alpha-aryl-substituted methionine derivatives, respectively, which function as AA- or AAX-mimetics. (C) 2000 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0968-0896(00)00138-3
文献信息
-
376. Substitution in arylsulphon-1- and -2-naphthalides作者:Raphael Consden、Joseph KenyonDOI:10.1039/jr9350001591日期:——
-
411. The nitration of phthalonaphthylimides and the facile preparation of 8-nitro-1-naphthylamine作者:Herbert H. Hodgson、J. Harold CrookDOI:10.1039/jr9360001844日期:——
-
Tarnowska, Societatis Scientiarum Lodziensis, Acta Chimica, 1957, vol. 2, p. 101,107作者:TarnowskaDOI:——日期:——
-
Chrzaszczewska et al., Societatis Scientiarum Lodziensis, Acta Chimica, 1958, vol. 3, p. 63,65作者:Chrzaszczewska et al.DOI:——日期:——
-
Tarnowska, Societatis Scientiarum Lodziensis, Acta Chimica, 1959, vol. 4, p. 143,144作者:TarnowskaDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
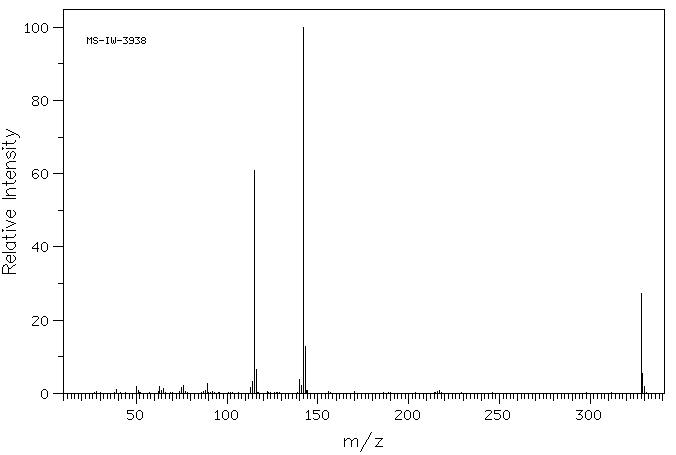
质谱MS
-
碳谱13CNMR
-
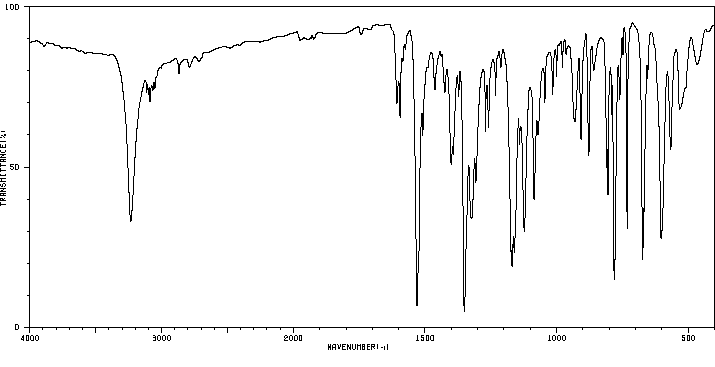
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮