2-亚甲基戊二腈 | 1572-52-7
中文名称
2-亚甲基戊二腈
中文别名
2,4-二氰基-1-丁烯;二亚甲基戊二腈
英文名称
2,4-dicyano-1-butene
英文别名
2-methyleneglutaronitrile;α-methyleneglutaronitrile;2-methylidenepentanedinitrile
CAS
1572-52-7
化学式
C6H6N2
mdl
MFCD00001869
分子量
106.127
InChiKey
NGCJVMZXRCLPRQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−9.6 °C(lit.)
-
沸点:254 °C(lit.)
-
密度:0.976 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
保留指数:1023
-
稳定性/保质期:
在常温常压下稳定,避免与强氧化剂和强碱接触。
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:47.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
危险类别码:R20/21/22
-
危险品运输编号:UN 3276 6.1/PG 3
-
WGK Germany:3
-
海关编码:2926909090
-
包装等级:III
-
危险类别:6.1
-
安全说明:S36
-
危险性防范说明:P261,P280,P301+P310+P330,P302+P352+P312+P361+P364,P304+P340+P311,P305+P351+P338+P337+P313
-
危险性描述:H301+H311+H331,H315,H319
-
储存条件:请将容器密封保存,并储存在阴凉、干燥的地方。
SDS
2-亚甲基戊二腈 修改号码:5
模块 1. 化学品
产品名称: 2-Methyleneglutaronitrile
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
急性毒性(经皮) 第3级
急性毒性(吸入) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吸入或皮肤接触或吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
2-亚甲基戊二腈 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。呼叫解毒中心/医生。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
立即去除/脱掉所有被污染的衣物。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持容器密闭。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-亚甲基戊二腈
百分比: >98.0%(GC)
CAS编码: 1572-52-7
俗名: 2,4-Dicyano-1-butene
分子式: C6H6N2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
2-亚甲基戊二腈 修改号码:5
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 98 °C/0.5kPa
闪点: 126°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.98
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂, 强碱
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氰化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
2-亚甲基戊二腈 修改号码:5
模块 11. 毒理学信息
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 3276
正式运输名称: 腈类, 液体, 有毒的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Methyleneglutaronitrile
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
急性毒性(经皮) 第3级
急性毒性(吸入) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吸入或皮肤接触或吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
2-亚甲基戊二腈 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。呼叫解毒中心/医生。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
立即去除/脱掉所有被污染的衣物。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持容器密闭。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-亚甲基戊二腈
百分比: >98.0%(GC)
CAS编码: 1572-52-7
俗名: 2,4-Dicyano-1-butene
分子式: C6H6N2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
2-亚甲基戊二腈 修改号码:5
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 98 °C/0.5kPa
闪点: 126°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.98
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂, 强碱
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氰化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
2-亚甲基戊二腈 修改号码:5
模块 11. 毒理学信息
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 3276
正式运输名称: 腈类, 液体, 有毒的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
反应信息
-
作为反应物:参考文献:名称:卤代吡啶I.由二聚丙烯腈制备3-卤代甲基吡啶摘要:卤代吡啶I.由二聚丙烯腈合成3-卤代甲基吡啶。DOI:10.1002/hlca.19760590121
-
作为产物:参考文献:名称:Electrochemistry and reverse pulse polarographic determination of 1,2-dibromo-2,4-dicyanobutane摘要:DOI:10.1021/ac00281a033
文献信息
-
Iron-Catalyzed Alkylazidation of 1,1-Disubstituted Alkenes with Diacylperoxides and TMSN<sub>3</sub>作者:Rongbiao Wei、Haigen Xiong、Changqing Ye、Yajun Li、Hongli BaoDOI:10.1021/acs.orglett.0c00969日期:2020.4.17An iron-catalyzed radical alkylazidation of electron-deficient alkenes is reported. Alkyl diacyl peroxides work as the alkyl source, and trimethylsilyl azide acts as the azido reservoir. This method features mild reaction conditions, wide substrate scope, and good functional group tolerance, providing a range of α-azido esters, an α-azido ketone, and an α-azido cyanide in high yields. These azides
-
Regioselective biocatalytic hydrolysis of (E,Z)-2-methyl-2-butenenitrile for production of (E)-2-methyl-2-butenoic acid作者:Eugenia C Hann、Amy E Sigmund、Susan K Fager、Frederick B Cooling、John E Gavagan、Michael G Bramucci、Sarita Chauhan、Mark S Payne、Robert DiCosimoDOI:10.1016/j.tet.2003.10.120日期:2004.1Acidovorax facilis 72W nitrilase catalyzed the regioselective hydrolysis of (E,Z)-2-methyl-2-butenenitrile, producing only (E)-2-methyl-2-butenoic acid with no detectable conversion of (Z)-2-methyl-2-butenenitrile. (E)-2-Methyl-2-butenoic acid, produced in aqueous solution as the ammonium salt, was readily separated from (Z)-2-methyl-2-butenenitrile, and isolated in high yield and purity. The combination
-
A convenient general synthesis of 3-substituted 2H-chromene derivatives作者:Perry T Kaye、Xolani W. NocandaDOI:10.1039/b201827f日期:2002.5.10Reactions of 2-hydroxybenzaldehydes and 2-hydroxy-1-naphthaldehydes with various activated alkenes under Baylis–Hillman conditions have been shown to proceed with regioselective cyclisation to afford the corresponding 3-substituted chromene derivatives. In some cases competitive dimerisation of the alkene component was observed, and direct dimerisation in the absence of the aldehyde has been explored.在Baylis-Hillman条件下,2-羟基苯甲醛和2-羟基-1-萘甲醛与多种活化烯烃反应,显示出区域选择性的环化,生成相应的3-取代色烯衍生物。在某些情况下,观察到烯烃组分的竞争性二聚作用,并且在没有醛的情况下直接二聚也已被探索。
-
Intramolecular meerwein reactions of the anthraquinone system. Synthesis, X-ray structural analysis and spectroscopic properties of anthra[9,1-<i>bc</i>:10,5-<i>b'c'</i>]tetrahydrodipyran derivatives作者:H. Fritz、G. Rihs、P. Sutter、C. D. WeisDOI:10.1002/jhet.5570180817日期:1981.12methylphosphonate as reaction medium. Compounds 5–18 were isolated as mixtures of diastereomers, some of which were separated by crystallization. Proof of structure and stereochemistry was obtained for some of the compounds by X-ray crystallographic analysis. The 1H- and 13C-nmr data provided further support. Surprisingly large chemical shift differences between some of the proton signals of isomers were observed1,5-蒽醌双(硫酸氢二重氮)与充分活化的烯烃(如丙烯腈,丙烯酸酯,甲基丙烯腈及其酯,苯乙烯和α-甲基苯乙烯)的Meerwein反应产生了新杂环系统蒽的衍生物[9,1- bc:10,5 - b'c' ]-2,3,7,8-四氢双吡喃。通过使用甲基膦酸二甲酯作为反应介质,可以实现衍生物的分离并提高产率。化合物5-18是非对映异构体的混合物,其中一些通过结晶分离。通过X射线晶体学分析获得了某些化合物的结构和立体化学证明。的1 H-和13C-nmr数据提供了进一步的支持。尽管两个不对称中心通过键和空间相隔很远,但观察到异构体的某些质子信号之间的化学位移差异出乎意料地大。基于电荷转移络合物的氧化还原调节来解释其形成机理,其中脂族基团攻击蒽醌的氧原子。提出将这种新型反应分类为分子内Meerwein反应。
-
Pd-Mediated C-C Bond Formation with Olefins and Acetylenes on Solid Support: A Scope and Limitations Study作者:Sabine Berteina、Sebastian Wendeborn、Wolfgang K.-D. Brill、Alain De MesmaekerDOI:10.1055/s-1998-22662日期:1998.6In this paper we report the scope and limitations of Pd(0)-mediated coupling reactions between aromatic iodides linked to a polystyrene resin and terminal acetylenes and olefins (Heck reactions). Optimized reaction conditions were evaluated with a number of different reagents. The optimized reaction conditions were frequently found to be superior to those previously reported in the literature and resulted in excellent yields of the products upon cleavage from the solid phase.
表征谱图
-
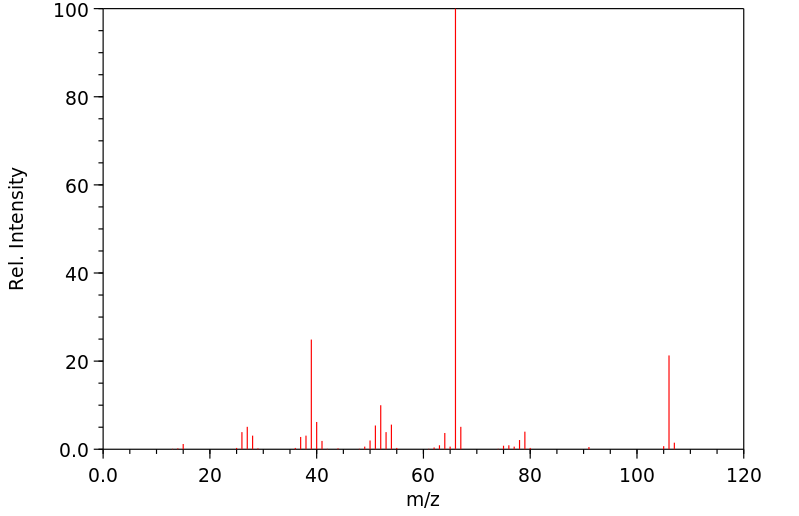
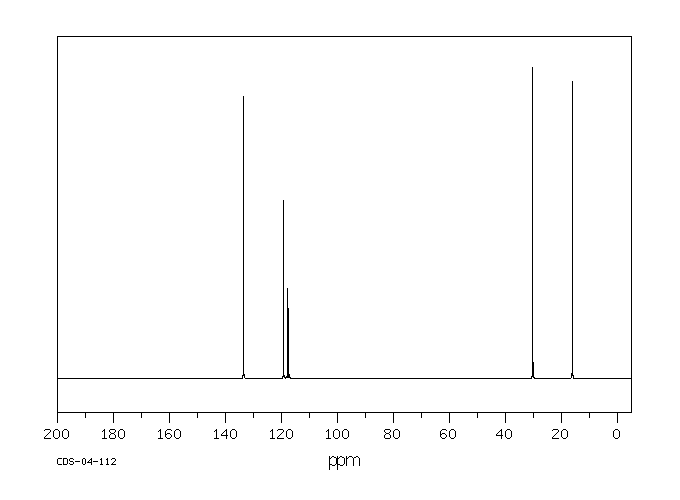
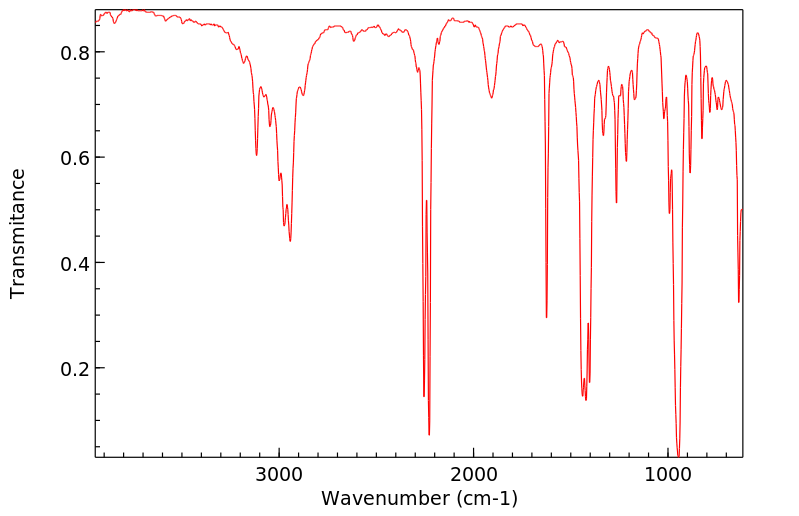
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷