2-氯-3-吡咯烷基-1,4-萘醌 | 59641-25-7
中文名称
2-氯-3-吡咯烷基-1,4-萘醌
中文别名
——
英文名称
2-chloro-3-(pyrrolidin-1-yl)-1,4-naphthoquinone
英文别名
2-Chloro-3-pyrrolidino-1,4-naphthoquinone;2-chloro-3-(pyrrolidin-1-yl)naphthalene-1,4-dione;2-chloro-3-pyrrolidin-1-ylnaphthalene-1,4-dione
CAS
59641-25-7
化学式
C14H12ClNO2
mdl
——
分子量
261.708
InChiKey
QWKZKEUBFVSTDB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:101-103 °C(lit.)
-
沸点:368.1±42.0 °C(Predicted)
-
密度:1.38±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:18
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:37.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3-二氯-1,4-萘醌 2,3-Dichloro-1,4-naphthoquinone 117-80-6 C10H4Cl2O2 227.047 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(phenylthio)-3-(pyrrolidin-1-yl)naphthalene-1,4-dione 500755-47-5 C20H17NO2S 335.426
反应信息
-
作为反应物:描述:2-氯-3-吡咯烷基-1,4-萘醌 以 甲醇 为溶剂, 生成参考文献:名称:A general strategy for visible-light decaging based on the quinone cis-alkenyl lock摘要:将对羟基肉桂酸衍生物的快速热环化与醌的分子内光还原相结合,可得到新的可见光光解保护基,其吸收波长远超过450纳米。DOI:10.1039/c9cc01073d
-
作为产物:描述:参考文献:名称:“在水上”:在水悬浮液中与1,4-醌进行前所未有的亲核取代和加成反应摘要:在没有传统合成催化剂的催化剂的情况下,描述了水性悬浮液中1,4-醌与芳香胺,伯脂肪胺,氨基酸,氨基酸酯,杂环胺,肼,酰胺和硫醚的独特亲核取代和加成反应催化剂存在下在非水介质中进行这些反应的途径。DOI:10.1016/j.tetlet.2009.07.149
文献信息
-
2-(Pyrrolidin-1-yl)-1,4-naphthoquinone and 2-phenylsulfanyl-3-(pyrrolidin-1-yl)-1,4-naphthoquinone作者:Daniel E. Lynch、Ian McClenaghanDOI:10.1107/s0108270102019194日期:2002.12.15The structure of 2-(pyrrolidin-1-yl)-1,4-naphthoquinone, C(14)H(12.9)5Cl(0.05)NO(2), (I), is actually a 0.95:0.05 mixture including 2-chloro-3-(pyrrolidin-1-yl)-1,4-naphthoquinone as a minor impurity, but (I) was resolved as a single molecule containing a Cl atom with 5% occupancy at the 3-position. Compound (I) was prepared from the fully chloro-substituted analogue in an attempt to produce the disubstituted pyrrolidinyl derivative. 2-Phenylsulfanyl-3-(pyrrolidin-1-yl)1,4-naphthoquinone, C20H17NO2S, (II), was also prepared from 2-chloro-3-(pyrrolidin-1-yl)-1,4-naphthoquinone, using a strong exocyclic nucleophile. The structure of (II) differs from previous structures of 2,3-dichloro-1,4-naphthoquinone and its derivatives in that the naphthoquinone ring is non-planar.
-
‘On water’ assisted synthesis and biological evaluation of nitrogen and sulfur containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents作者:Vishnu K. Tandon、Hardesh K. Maurya、Manoj K. Verma、Rohitashw Kumar、Praveen K. ShuklaDOI:10.1016/j.ejmech.2010.02.023日期:2010.62-Chloro-3-(4-methylpiperazin-1-yl)naphthalene-1,4-dione (3a), 2-chloro-3-(pyrrolidin-1-yl)naphthalene-1,4-dione (3b), 2-chloro-3-(piperidin-1-yl)naphthalene-1,4-dione (3c), 2-chloro-3-morpholinonaphthalene-1,4-dione (3d), 2-chloro-3-(2-phenylhydrazinyl)naphthalene-1,4-dione (3e), 2-(allylamino)-3-chloronaphthalene-1,4-dione (3f), 2-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-ylthio)acetic acid (3g), 2-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-ylthio)succinic acid (3h), methyl 2-(3-chloro-1,4-choxo-1,4-dihydronaphthalen-2-ylthio)acetate (3i), 2-chloro-3-(2-mercaptoethylthio)naphthalene-1,4-dione (3j), 3-hydroxy-4-methyl-4H-naphtho[2,3-b][1,4]thiazine-5,10-dione (3k) and compounds 31-q have been synthesized by a green methodology approach using water as solvent and evaluated for their antifungal and antibacterial activity. The antifungal profile of 3a-n indicated that compounds 3a-d, 3j, 3e and 3k have potent antifungal activity. Amongst the most promising antifungal compounds, 3a-g, 3j, 3k showed better antifungal activity than clinically prevalent antifungal drugs Fluconazole and Amphotericin-B against Trichophyton mentagraphytes and compounds 3j and 3k have been found to be lead antifungal bicyclic and tricyclic 1,4-naphthoquinones. Compound 3k also exhibited pronounced antibacterial activity.
-
US5684035A申请人:——公开号:US5684035A公开(公告)日:1997-11-04
-
US5807898A申请人:——公开号:US5807898A公开(公告)日:1998-09-15
表征谱图
-
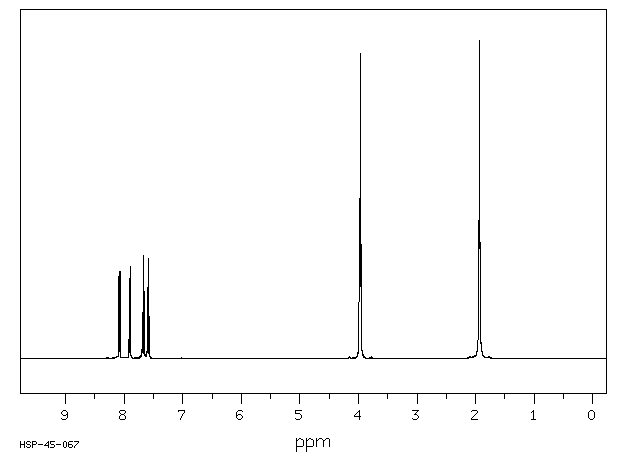
氢谱1HNMR
-
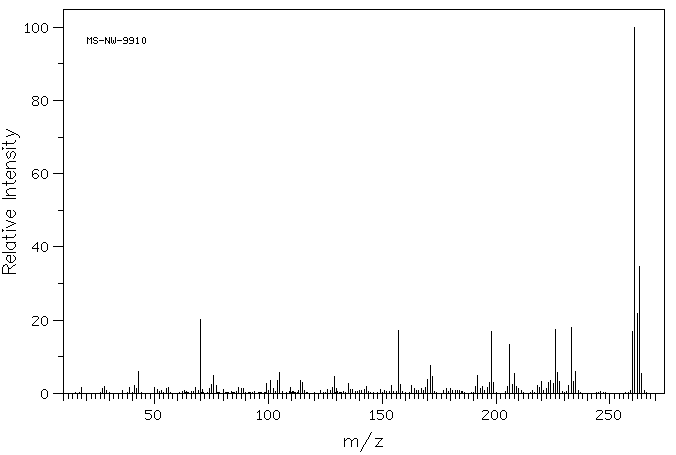
质谱MS
-
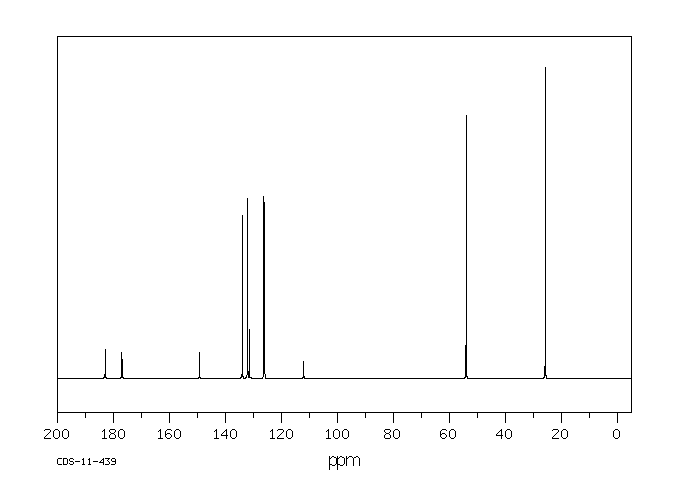
碳谱13CNMR
-
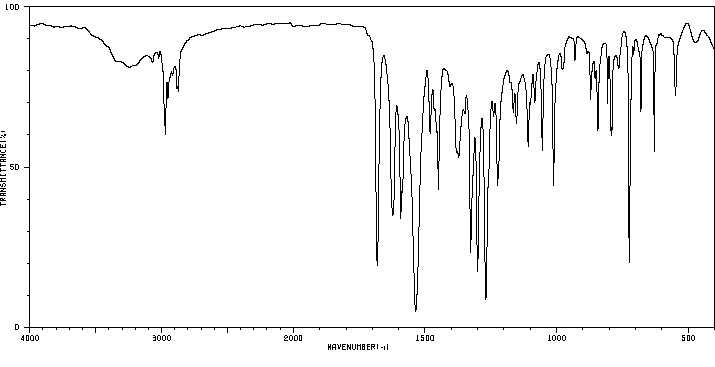
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮