2H-噻喃,2-乙基四氢- | 1613-52-1
中文名称
2H-噻喃,2-乙基四氢-
中文别名
——
英文名称
2-Aethyl-pentamethylensulfid
英文别名
2-Ethylthiacyclohexane;2-ethylthiane
CAS
1613-52-1
化学式
C7H14S
mdl
——
分子量
130.254
InChiKey
GYIFZSXJAVPBLL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:191°C (estimate)
-
密度:0.9257
-
保留指数:1024;1011;1011;1024
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Stereoselective Synthesis oftrans-2-Alkylthiane 1-Oxides摘要:2-烷基噻烷与叔丁基次氯酸盐反应生成反式-1-叔丁氧噻烷盐,这些盐在高立体选择性下水解为反式-2-烷基噻烷-1-氧化物。DOI:10.1055/s-1990-27088
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 sodium sulfide 作用下, 生成 2H-噻喃,2-乙基四氢-参考文献:名称:Hopkins; Rall, ACS Division of Petroleum Chemistry, Inc. Preprints, 1957, vol. 2, # 1, p. 231,233, 237摘要:DOI:
文献信息
-
Stereoselective synthesis of cis and trans n-tosyl sulphilimines and sulphoxides from 2-alkylthianes and 2-alkylthiolanes assignments of configurations作者:I. Jalsovszky、F. Ruff、M. Kajtár-Peredy、I. Kövesdi、Á. KucsmanDOI:10.1016/s0040-4020(01)88170-9日期:1986.1thiolane derivatives with 2-methyl, 2-ethyl, 2-isopropyl and 2-tert-butyl groups were prepared and converted to cis and trans sulphilimines and sulphoxides by various stereoselective methods. Cis-sulphilimines were formed by using t-BuOCl and TsNH- in a two-stage process, while cyclic sulphides were converted by chloramine-T predominantly to trans-sulphilimines. Sulphoxides enriched in cis and trans isomers
-
PROCESS FOR PREPARING AMIC ACID ESTERS申请人:——公开号:US20030032667A1公开(公告)日:2003-02-13The present invention aims at providing a process for producing an amic acid ester useful as an intermediate for agrochemical, easily at a low cost industrially. The present invention provides a process for producing an amic acid ester represented by the following general formula (7): 1 (wherein A is a substituted or unsubstituted lower alkylene group or the like; R 1 is a substituted or unsubstituted lower alkyl group or the like; and R 3 is a hydrogen atom or a lower alkyl group), which process comprises reacting, in the presence of water, an amino acid represented by the following general formula (1): 2 (wherein A has the same definition as given above) with a halogenated carbonic acid ester represented by the following general formula (2): 3 (wherein R 1 has the same definition as given above and X is a halogen atom) to form an amide compound represented by the following general formula (3): 4 (wherein A and R 1 have the same definitions as given above), then reacting the amide compound with a halogenated carbonic acid ester represented by the following general formula (4): 5 (wherein R 2 is a substituted or unsubstituted lower alkyl group or the like; and X is a halogen atom) to form, in the system, a mixed acid anhydride represented by the following general formula (5): 6 (wherein A, R 1 and R 2 have the same definitions as given above), and reacting the mixed acid anhydride with an amine compound represented by the following general formula (6): 7 (wherein R 3 has the same definition as given above and Het is a substituted or unsubstituted heterocyclic group).本发明旨在提供一种用于农药中间体的制备的amic酸酯的工艺,该工艺在工业上易于低成本实现。本发明提供一种制备由下列通式(7)表示的amic酸酯的工艺:1(其中A是取代或未取代的较低烷基等;R1是取代或未取代的较低烷基等;R3是氢原子或较低烷基等),该工艺包括在水的存在下,将由下列通式(1)表示的氨基酸:2(其中A具有如上定义)与由下列通式(2)表示的卤代碳酸酯:3(其中R1具有如上定义,X是卤素原子)反应,形成由下列通式(3)表示的酰胺化合物:4(其中A和R1具有如上定义),然后将酰胺化合物与由下列通式(4)表示的卤代碳酸酯:5(其中R2是取代或未取代的较低烷基等;X是卤素原子)在体系中反应,形成由下列通式(5)表示的混合酸酐:6(其中A、R1和R2具有如上定义),并将混合酸酐与由下列通式(6)表示的胺化合物:7(其中R3具有如上定义,Het是取代或未取代的杂环基)反应。
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Ag: MVol.B7, 1.20.3.5, page 83 - 93作者:DOI:——日期:——
-
Rozen, A. M.; Nikitina, V. S.; Lyapina, N. K., Radiokhimiya, 1972, vol. 14, p. 597 - 602作者:Rozen, A. M.、Nikitina, V. S.、Lyapina, N. K.DOI:——日期:——
-
GORDADZE G. N.; BARYKINA L. R.; BEZINGER N. N.; BRODSKIJ E. S.; VOLYNSKIJ+, ORGAN. SOEDIN. SERY. T. 2. RIGA, 1980, 314-325作者:GORDADZE G. N.、 BARYKINA L. R.、 BEZINGER N. N.、 BRODSKIJ E. S.、 VOLYNSKIJ+DOI:——日期:——
表征谱图
-
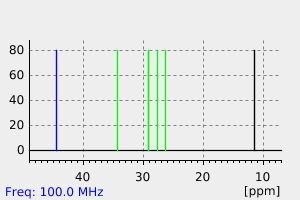
氢谱1HNMR
-
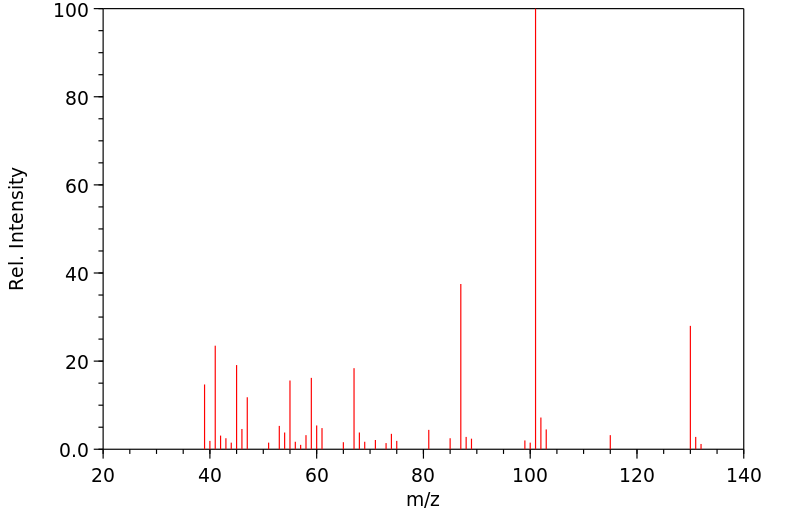
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿普卡林
硫化环戊烷
硫代环己酮
甲基硫酸四氢1-乙基-2-[1,2,3,4--1-(2-羟基乙基)-2,2,4-三甲基-6-喹啉基]苯[cd]吲哚正离子
甲基(1,1-二氧化四氢-2h-噻喃-4-基)醋酸盐
外-3-乙酰基-2-硫杂二环<2.2.2>辛-5-烯
四氢硫代吡喃-4-胺盐酸盐
四氢硫代吡喃-4-羰酰氯
四氢硫代吡喃-4-羧酸甲酯
四氢硫代吡喃-4-甲腈
四氢硫代吡喃-4-基甲醇
四氢硫代吡喃-3-甲醛
四氢噻喃-4-醇
四氢噻喃-4-酮肟
四氢噻喃-4-酮 1,1-二氧化物
四氢噻喃-4-酮
四氢噻喃-4-胺
四氢噻喃-4-肼双盐酸盐
四氢噻喃-4-甲醛
四氢-4H-硫代吡喃-4-酮 1-氧化物
四氢-4-氧代-2H-噻喃-3-甲酸甲酯
四氢-3-甲基-2H-噻喃
四氢-3-氧代-6H-噻喃-2-甲酸甲酯
四氢-2H-硫代吡喃-4-醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-羧醛 1,1-二氧化物
四氢-2H-硫代吡喃-4-甲醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-乙醛
四氢-2H-噻喃-4-羧酸甲酯1,1-二氧化物
四氢-2H-噻喃-4-甲酰肼
四氢-2H-噻喃-4-甲腈1,1-二氧化
四氢-2H-噻喃-3-醇1,1-二氧化物
四氢-2H-噻喃-3-羧酸1,1-二氧化物
四氢-(9ci)-2H-硫代吡喃-4-羧酸
噻-4-基甲胺
叔-丁基[(1S,2R)-1-苯甲基-2-羟基-3-[异丁基[(4-硝基苯基)磺酰]氨基]丙基]氨基甲酸酯
二氢-5,5-二甲基-2H-硫基吡喃-3(4H)-酮-1,1-二氧化物
二氢-2H-硫代吡喃-3(4h)-酮
二氢-2H-硫代吡喃-3(4H)-酮-1,1-二氧化物
乙酸四氢-2H-噻喃-2-基酯
三环己基乙基硼酸钠
n-[四氢-2H-硫代吡喃-4-基]氨基甲酸-1,1-二甲基乙酯
N-甲基四氢-2H-硫代吡喃-4-胺盐酸盐
N-甲基四氢-2H-噻喃-4-胺盐酸盐
N-甲基(四氢硫代吡喃-4-基)甲基胺
9-硫杂二环[3.3.1]壬烷-2,6-二酮
9-硫杂二环[3.3.1]壬烷
8-硫杂二环[3.2.1]辛烷-3-酮
8-硫杂-2,3-二氮杂螺[4.5]癸烷
8-乙烯基-7-硫杂-二环[4.2.0]辛烷
7-硫杂-2-氮杂螺[3.5]壬烷半草酸酯