甲基烯丙基氰化物 | 4786-19-0
中文名称
甲基烯丙基氰化物
中文别名
——
英文名称
3-methyl-3-butene nitrile
英文别名
methallyl cyanide;3-methylbut-3-enenitrile
CAS
4786-19-0
化学式
C5H7N
mdl
——
分子量
81.1173
InChiKey
OIQDAVBXDLGCID-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:136.2-136.4 °C
-
密度:0.816±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
SDS
反应信息
-
作为反应物:描述:甲基烯丙基氰化物 在 lithium diethylamide 作用下, 以 四氢呋喃 为溶剂, 反应 19.0h, 以75%的产率得到3,5,5-Trimethyl-1-amino-4-cyanocyclohexa-1,3-diene参考文献:名称:3-甲基-3-丁烯腈的新型环二聚。有效合成4-氰基异佛尔酮摘要:3-甲基-3-丁烯腈(1)被二烷基酰胺锂二聚生成选择性的3,5,5-三甲基-1-氨基-4-氰基环己-1,3-二烯(2),很容易被酸水溶液水解为定量提供4-氰基异佛尔酮(3,5,5-三甲基-4-氰基-环己-2-烯酮)(3)。DOI:10.1016/0040-4039(80)80206-1
-
作为产物:描述:参考文献:名称:The Decarboxylation of α,β-Unsaturated Malonic Acid Derivatives via β,γ-Unsaturated Intermediates. II. The Effect of α-Substituents upon Product Composition and Rate摘要:DOI:10.1021/ja01101a046
文献信息
-
Enantioselective Hydrolysis of Functionalized 2,2-Disubstituted Oxiranes with Bacterial Epoxide Hydrolases作者:Andreas Steinreiber、Ingrid Osprian、Sandra F. Mayer、Romano V. A. Orru、Kurt FaberDOI:10.1002/1099-0690(200011)2000:22<3703::aid-ejoc3703>3.0.co;2-3日期:2000.11ether-oxygen to the stereogenic quaternary carbon center of the oxirane ring had a profound influence on the enantioselectivity, and several oxiranes were resolved with good to excellent selectivities. The enantiomerically enriched epoxides and vicinal diols thus obtained contain a useful “synthetic handle” in their side chain, which allows their use as building blocks in asymmetric synthesis.
-
Synthesis of γ-hydroxy α,β-unsaturated amides by base-induced isomerization of epoxy amides作者:Peter B. Brooks、Charles M. MarsonDOI:10.1016/s0040-4020(98)00519-5日期:1998.8Treatment of 3,4-epoxyamides with LDA affords γ-hydroxy-α,β-unsaturated amides, usually with high (E)-selectivity. The 3,4-epoxyamides were prepared by the epoxidation of β,γ-unsaturated amides with meta-chloroperbenzoic acid.
-
Incorporation of Trifluoroisoleucine into Proteins in Vivo作者:Pin Wang、Yi Tang、David A. TirrellDOI:10.1021/ja0298287日期:2003.6.1Two fluorinated derivatives of isoleucine: d,l-2-amino-3-trifluoromethyl pentanoic acid (3TFI, 2) and d,l-2-amino-5,5,5-trifluoro-3-methyl pentanoic acid (5TFI, 3) were prepared. 5TFI was incorporated into a model target protein, murine dihydrofolate reductase (mDHFR), in an isoleucine auxotrophic Escherichia coli host strain suspended in 5TFI-supplemented minimal medium depleted of isoleucine. Incorporation异亮氨酸的两种氟化衍生物:d,l-2-amino-3-trifluoromethyl pentanoic acid (3TFI, 2) 和 d,l-2-amino-5,5,5-trifluoro-3-methylpentanoic acid (5TFI, 3 ) 准备好了。5TFI 被纳入模型靶蛋白,鼠二氢叶酸还原酶 (mDHFR),在异亮氨酸营养缺陷型大肠杆菌宿主菌株中,悬浮在缺乏异亮氨酸的 5TFI 补充的基本培养基中。通过蛋白质产物的胰蛋白酶肽分析和基质辅助激光解吸电离质谱 (MALDI-MS) 证实了 5TFI 的掺入。氨基酸分析表明,超过 93% 的编码异亮氨酸残基被 5TFI 取代。大肠杆菌异亮氨酰-tRNA 合成酶 (IleRS) 对 5TFI 的活化率的测量产生了比异亮氨酸低 134 倍的特异性常数 (k(cat)/K(m))。5TFI 在编码的异亮氨酸位置成功引入细胞因子鼠白细胞介素
-
Raman and infrared spectra, conformational stability, barriers to internal rotation, vibrational assignment, and ab initio calculations of 3-methyl-3-butenenitrile作者:James R. Durig、Chao Zheng、Gamil A. Guirgis、Huimin Zhen、Andrea S. Drew、Douglas T. DurigDOI:10.1016/j.molstruc.2005.10.015日期:2006.3conformer based on infrared band contours, relative intensities, depolarization ratios and group frequencies. Several of the fundamentals for the gauche conformer have also been identified. The vibrational assignments are supported by normal coordinate calculations utilizing ab initio force constants. Complete equilibrium geometries have been obtained for both rotamers by ab initio calculations employing the摘要 液体和固体的拉曼 (3500–30 cm -1 ) 光谱以及气体和固体 3-甲基-3-丁烯腈、CH 2 C(CH 3 )CH 2 CN 的红外 (3500–40 cm -1 ) 光谱,已记录。已在流体相中鉴定出顺式和 gauche 构象异构体,但只有顺式形式保留在固体中。已经对溶解在液态氙中的样品的红外光谱进行了变温(-55 至 -100 °C)研究。根据这些数据,已确定焓差为163±16 cm -1 (1.20±0.19 kJ mol -1 ),顺式构象异构体是更稳定的旋转异构体。据估计,在 25°C 时存在 48±2% 的 gauche 构象异构体。提出了基于红外波段轮廓、相对强度、去极化率和群频率的顺式构象异构体的完整振动分配。还确定了 gauche 构象异构体的几个基本原理。使用 ab initio 力常数的法向坐标计算支持振动分配。通过使用 6-31G(d)、6-311G(d
-
Tetrahydro-1,5-benzoxazepines and tetrahydro-1<i>H</i>-1,5-benzodiazepines by a tandem reduction-reductive amination reaction作者:Richard A. Bunce、Christopher L. Smith、Jason R. LewisDOI:10.1002/jhet.5570410617日期:2004.11A tandem reduction-reductive amination reaction has been applied to the synthesis of (±)-4-alkyl-2,3,4,5-tetrahydro-1,5-benzoxazepines and (±)-4-alkyl-1-benzoyl-2,3,4,5-tetrahydro-1H-1,5-benzodiazepines. The nitro aldehydes and ketones required for 1,5-benzoxazepine ring closures were prepared by nucleophilic aromatic substitution of the alkoxides from several 3-buten-1-ol derivatives with 2-fluoro-1-nitrobenzene串联还原-还原胺化反应已用于合成(±)-4-烷基-2,3,4,5-四氢-1,5-苯并x氮平和(±)-4-烷基-1-苯甲酰基- 2,3,4,5-四氢-1 H -1,5-苯并二氮杂s。通过用2-氟-1-硝基苯将几种3-丁烯-1-醇衍生物中的醇盐进行亲核芳族取代,然后进行臭氧分解,可制备1,5-苯并x杂庚环闭环所需的硝基醛和酮。1,5-苯并二氮杂卓的前体是通过类似地添加N来制备的-(3-丁烯基)苯甲酰胺阴离子生成2-氟-1-硝基苯,然后进行臭氧分解。然后使用5%钯碳的甲醇溶液催化硝基羰基化合物的加氢反应,然后通过串联还原-还原胺化顺序得到目标杂环。色谱纯化后,高产率地分离出1,5-苯并x氮平。直接从氢化混合物中分离出固体形式的1,5-苯并二氮杂并在两个氮原子上具有不同的官能度。
表征谱图
-
氢谱1HNMR
-
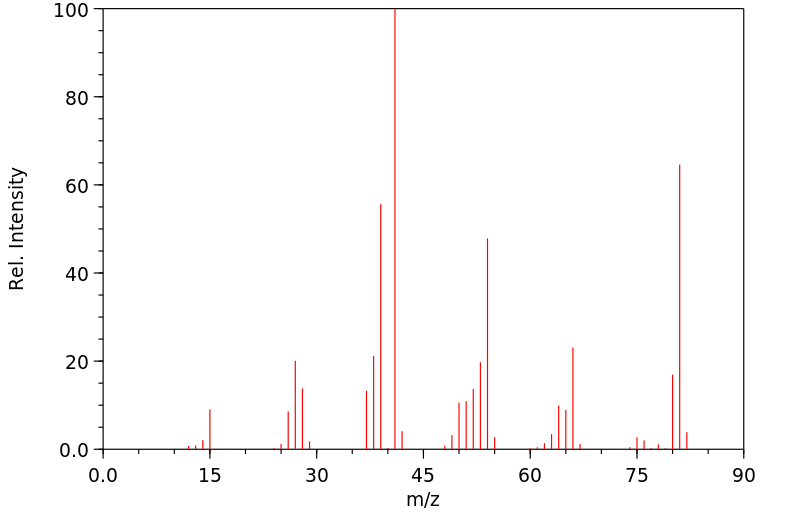
质谱MS
-
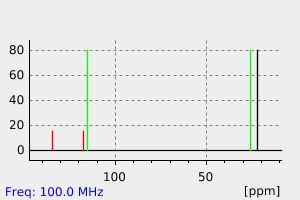
碳谱13CNMR
-
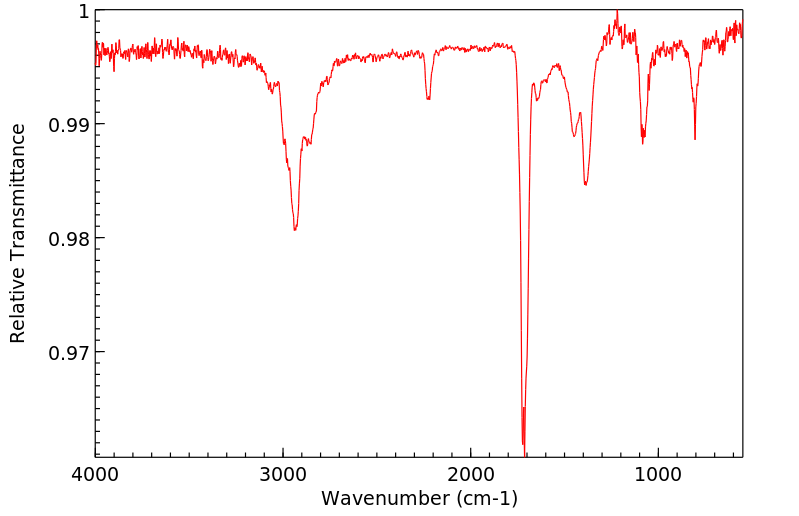
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷