1,8-二(苯氧基甲基)-萘 | 123036-57-7
中文名称
1,8-二(苯氧基甲基)-萘
中文别名
——
英文名称
1,8-bis(phenoxymethyl)-naphthalene
英文别名
1,8-bis(phenoxymethyl)naphthalene
CAS
123036-57-7
化学式
C24H20O2
mdl
——
分子量
340.422
InChiKey
FTMLSOIXAQEKOJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:26
-
可旋转键数:6
-
环数:4.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:1,8-二(苯氧基甲基)-萘 以 环己烷 为溶剂, 生成参考文献:名称:Ouchi, Akihiko; Koga, Yoshinori; Alam, Maksudul M., Journal of the Chemical Society. Perkin transactions II, 1996, # 8, p. 1705 - 1710摘要:DOI:
-
作为产物:描述:1,8-萘二甲酸酐 在 lithium aluminium tetrahydride 、 氢溴酸 、 potassium carbonate 作用下, 以 丙酮 为溶剂, 生成 1,8-二(苯氧基甲基)-萘参考文献:名称:Ouchi, Akihiko; Koga, Yoshinori; Alam, Maksudul M., Journal of the Chemical Society. Perkin transactions II, 1996, # 8, p. 1705 - 1710摘要:DOI:
文献信息
-
Laser-jet photolysis of 1,8-bis(substituted-methyl)naphthalenes; the effect of heteroatom leaving groups on the two-photon C–C bond formation作者:Akihiko Ouchi、Waldemar AdamDOI:10.1039/c39930000628日期:——The formation of acenaphthene 4 by laser-jet photolysis of 1,8-bis(phenoxyrmethyl)-(1a), 1,8-bis(phenylthiomethyl)-(1b) and 1,8-bis(phenylselenomethyl)-naphthalene (1c) strongly depends on the hetroatom of the leaving group; the increasing order of naphthalene consumption was 1câ1b > 1a, whereas that for the formation of 4 was 1c > 1a > 1b.
-
Highly efficient CC bond formation in the two-photon chemistry of 1,8-bis(substituted-methyl)naphthalenes by direct excitation of photoactive leaving groups作者:Akihiko Ouchi、Akira Yabe、Waldemar AdamDOI:10.1016/s0040-4039(00)73419-8日期:1994.8High efficiency in the two-photon intramolecular C-C bond formation to the two-photon product acenaphthene (4), obtained in a maximum yield of 72%, was achieved by direct activation of two separate leaving groups of 1,8-bis(phenoxymethyl)-(1a), 1,8-bis(phenylthiomethyl)-(1b), and 1,8-bis(phenylselenomethyl)-naphthalenes (1c) during KrF excimer laser (248 nm) and of 1,8-bis(4-benzoylphenoxymethyl)-(1d), 1,8-bis-(3-benzoylphenoxymethyl)-(1e), and 1,8-bis(2-naphthoxymethyl)naphthalenes (1f) during XeF excimer laser (351 nm) photolyses.
-
Time-delayed, two-color excimer laser photolysis of 1,8-bis(substituted-methyl)naphthalenes with group 16 atom leaving groups作者:Akihiko Ouchi、Yoshinori KogaDOI:10.1016/0040-4039(95)01970-s日期:1995.12Time-delayed, two-color photolysis of 1,8-bis(phenoxymethyl)-(1a), 1,8-bis(phenylthiomethyl)-(1a), and 1,8-bis(phenylselenomethyl)-naphthalene (1c) was conducted by successive irradiation of XeCl (308 nm) and XeF (351 nm) excimer lasers. The yield of the two-photon product, acenaphthene 3, was strongly dependent on the delay time and showed two maxima at different delay times.
表征谱图
-
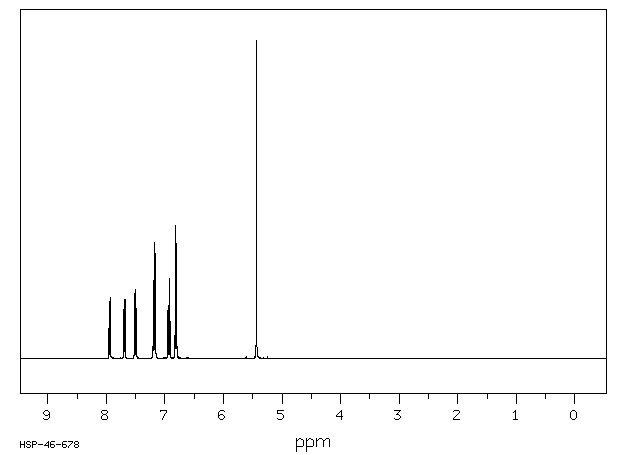
氢谱1HNMR
-
质谱MS
-
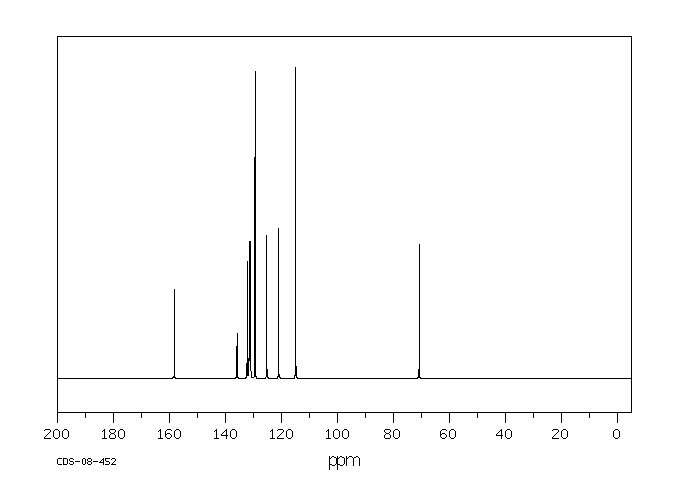
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮