4-(二甲氨基)安息香 | 6317-85-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:161-163 °C(lit.)
-
沸点:439.6±35.0 °C(Predicted)
-
密度:1.171±0.06 g/cm3(Predicted)
-
保留指数:2410
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:40.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S26,S37,S39
-
海关编码:2922509090
-
危险品标志:Xi
-
危险类别码:R36/37/38
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-[4-(Dimethylamino)phenyl-2-hydroxy-1-phenylethanone 33458-29-6 C16H17NO2 255.316 安息香精油 2-hydroxy-2-phenylacetophenone 119-53-9 C14H12O2 212.248 —— 1-(4-dimethylaminophenyl)-2-phenyl-1,2-ethanedione 22711-20-2 C16H15NO2 253.301 2-羟基-1-(4-甲氧基苯基)-2-苯乙酮 4-methoxybenzoin 4254-17-5 C15H14O3 242.274 —— 2-(benzo[d][1,3]dioxol-5-yl)-2-hydroxy-2-phenylethanone 36715-46-5 C15H12O4 256.258 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-[4-(Dimethylamino)phenyl-2-hydroxy-1-phenylethanone 33458-29-6 C16H17NO2 255.316 4-二甲基氨基-2-苯基苯乙酮 α-phenyl-(4-N,N-dimethylamino)acetophenone 97606-39-8 C16H17NO 239.317 —— 2-(4-(dimethylamino)phenyl)-1-phenylethan-1-one 6266-95-1 C16H17NO 239.317 —— 1-(4-dimethylaminophenyl)-2-phenyl-1,2-ethanedione 22711-20-2 C16H15NO2 253.301
反应信息
-
作为反应物:描述:参考文献:名称:Recent advances in P450 research摘要:P450 酶由含血红素的蛋白质超家族组成,可催化结构不同的化学物质的氧化代谢。在过去的几年里,P450研究在许多方面都取得了重大进展,所获得的信息目前正在应用于药物开发和临床实践。最近,通过晶体学确定了哺乳动物 P450 的结构,这是一项重大成就。这些研究的结果将对理解 P450 酶的结构-活性关系并促进药物相互作用的预测产生重大影响。此外,新技术还促进了几个新的 P450 等位基因的鉴定。这些信息将深刻影响我们对药物反应个体差异原因的理解,并将这些差异与许多治疗药物的功效或毒性联系起来。最后,最近在构建 P450 无效动物方面取得的成就已经确定了这些酶在毒性中的作用。此外,人源化转基因动物和植物的构建也取得了进展。总体而言,P450 领域的最新发展成果将是更安全、更有效的药物疗法。DOI:10.1038/sj.tpj.6500044
-
作为产物:描述:2-[4-(Dimethylamino)phenyl-2-hydroxy-1-phenylethanone 反应 0.17h, 以95%的产率得到4-(二甲氨基)安息香参考文献:名称:Mass-spectral behavior and thermal stability of hetaryl analogs of unsymmetrical benzoins摘要:DOI:10.1007/s10593-006-0110-6
文献信息
-
IRON(III)-ETHYLENEDIAMINETETRA-ACETIC ACID MEDIATED AEROBIC OXIDATION OF α-HYDROXYKETONES: A SIMPLE AND CONVENIENT SYNTHESIS OF α-DIKETONES作者:T. Venkateshwar Rao、Rajendra S. Dongre、Suman L. Jain、Bir SainDOI:10.1081/scc-120006026日期:2002.1ABSTRACT Iron(III)-ethylenediaminetetraacetic acid in aqueous methanol offers a simple, environmentally acceptable synthetic tool to oxidize α-hydroxyketones α-diketones with molecular oxygen, in excellent yields and under mild conditions and without any side reactions.
-
Electrochemical Oxidation of Benzoins to Benzils in the Presence of a Catalytic Amount of KI in Basic Media作者:Mitsuhiro Okimoto、Yukio Takahashi、Yuji Nagata、Gaku Sasaki、Kaori NumataDOI:10.1055/s-2005-861809日期:——Various benzoins were transformed into the corresponding benzils via an electrochemical method. Although the strategy of oxidizing benzoins to the corresponding benzils is generally chosen depending on the nature of the aromatic moiety, the electrochemical technique reported herein allows oxidation that is widely applicable, and without the use of environmentally undesirable oxidants.
-
Chromatographic enantioseparation by poly(biphenylylacetylene) derivatives with memory of both axial chirality and macromolecular helicity作者:Ryoma Ishidate、Tomoyuki Ikai、Shigeyoshi Kanoh、Eiji Yashima、Katsuhiro MaedaDOI:10.1002/chir.22687日期:2017.3with a chiral alcohol and both the induced main‐chain helicity and the pendant axial chirality were maintained, that is, memorized, after complete removal of the chiral alcohol. The stability of the helicity memory of the poly‐Ac's in a solution was lower than that of the analogous poly(biphenylylacetylene)s bearing two methoxymethoxy groups at the 2‐ and 2′‐positions of the biphenyl pendants (poly‐MOM's)新型的联苯撑乙炔通过聚合相应的联苯乙炔,合成了在联苯侧基(poly- Ac 's)的2和2'位置带有两个乙酰氧基和在4'-位置带有烷氧羰基的新型聚(联苯基乙炔)衍生物。铑催化剂。将所得到的有规立构(顺式- transoidal)聚AC通过与手性醇的非共价相互作用折叠成主要为单手螺旋构型并伴有联苯侧基的优选单手轴向扭曲构型,并保持了诱导的主链螺旋性和侧基轴向手性记住,完全除去手性酒精后。溶液中聚Ac的螺旋记忆的稳定性低于在联苯侧基的2和2'位置带有两个甲氧基甲氧基的类似聚(联苯乙炔)的稳定性(poly- MOM ' s)。但是,在固态状态下,poly- Ac的螺旋度记忆的是更稳定的,表现出对几种外消旋体比以前报道的聚更好的手性识别能力MOM作为高效液相色谱法在手性固定相使用时。尤其是,具有螺旋记忆的基于聚Ac的CSP有效地将外消旋安息香衍生物分离为对映体。
-
Reactions of azulenes with 1,2-diaryl-1,2-ethanediols in methanol in the presence of hydrochloric acid: comparative studies on products, crystal structures, and spectroscopic and electrochemical properties作者:Miwa Nakatsuji、Yasuhito Hata、Takeshi Fujihara、Kensaku Yamamoto、Masato Sasaki、Hideko Takekuma、Masakuni Yoshihara、Toshie Minematsu、Shin-ichi TakekumaDOI:10.1016/j.tet.2004.05.022日期:2004.7Similarly, reaction of methyl azulene-1-carboxylate (1b) with 2b under the same reaction conditions as 1a gives no product; however, reactions of 1-chloroazulene (1c) and the parent azulene (1d) with 2b under the same reaction conditions as 1a give 2-[3-(1-chloroazulenyl)]-1,1-bis(4-methoxyphenyl)ethylene (4) (81% yield) and 2-azulenyl-1,1-bis(4-methoxyphenyl)ethylene (5) (15% yield), respectively. Along尽管愈创木酚(1a)与1,2-二苯基-1,2-乙二醇(2a)在甲醇中,在60°C的盐酸中,在有氧条件下于有氧条件下反应3小时没有产生任何产物,但1a与1, 2-双(4-甲氧基苯基)-1,2-乙二醇(2b)在与2a相同的反应条件下得到新的乙烯衍生物2-(3-愈创木烯基)-1,1-双(4-甲氧基苯基)乙烯(3),产率为97%。类似地,在与1a相同的反应条件下,将z蓝色-1-羧酸甲酯(1b)与2b反应,没有产物。但是,1-氯杂氮烯(1c)与母体杂氮烯(1d)与2b在与1a相同的反应条件下得到2- [3-(1-氯氮杂烯基)]-1,1-双(4-甲氧基苯基)乙烯(4)(81%收率)和2-氮杂烯基-1, 1-双(4-甲氧基苯基)乙烯(5)(15%收率)。除上述反应外,1a与1,2-双(4-羟苯基)-1,2-乙二醇(2c)和1- [4-(二甲基氨基)苯基] -2-苯基-1,2-乙二醇的反应(2d)在与2b
-
Influence of protonation of peripheral substituents on photophysical and photochemical properties of tetrapyrazinoporphyrazines作者:Veronika Novakova、Eva H. Mørkved、Miroslav Miletin、Petr ZimcikDOI:10.1142/s1088424610002458日期:2010.7
Octasubstituted zinc tetrapyrazinoporphyrazines with four N,N-dimethylaminophenyls and four phenyl or pyridin-3-yl substituents were synthesized and fully characterized. Their fluorescence quantum yields in DMF or pyridine were very low, almost undetectable, as a consequence of ultrafast intramolecular charge transfer. Titration of their DMF solutions with sulfuric acid led to increase of the fluorescence quantum yields by two orders of magnitude when the full protonation of peripheral substituents was achieved. Intramolecular charge transfer is no longer a favorable way of excited-state relaxation at full protonation of N,N-dimethylaminophenyl substituents because of loss of donor centers (free electron pair on its nitrogen). Similarly, singlet oxygen quantum yields also increased by two orders of magnitude when sulfuric acid was added to tetrapyrazinoporphyrazine solutions in DMF. Protonation at azomethine nitrogens of tetrapyrazinoporphyrazine macrocycle was observed at higher acid concentrations and it led to considerable decrease of fluorescence quantum yields. Octaphenyl zinc tetrapyrazinoporphyrazine and octa(pyridin-3-yl) zinc tetrapyrazinoporphyrazine were used as controls without intramolecular charge transfer. Their fluorescence and singlet oxygen quantum yields were high in DMF and decreased at higher concentrations of sulfuric acid due to protonation of azomethine nitrogens. The results suggest that the photophysical and photochemical properties of studied compounds may be controlled by changes of pH of medium.
我们合成了具有四个 N,N-二甲基氨基苯基和四个苯基或吡啶-3-基取代基的八代四吡嗪卟啉锌,并对其进行了全面表征。由于超快的分子内电荷转移,它们在 DMF 或吡啶中的荧光量子产率非常低,几乎检测不到。当外围取代基完全质子化时,用硫酸滴定它们的 DMF 溶液可使荧光量子产率提高两个数量级。在 N,N-二甲氨基苯基取代基完全质子化时,分子内电荷转移不再是激发态弛豫的有利方式,因为供体中心(其氮上的自由电子对)已经丧失。同样,在 DMF 中的四吡嗪卟嗪溶液中加入硫酸后,单线态氧量子产率也增加了两个数量级。在酸浓度较高时,四吡嗪卟啉大环的偶氮甲基硝基会发生质子化,从而导致荧光量子产率大幅下降。八苯基四吡嗪卟嗪锌和八(吡啶-3-基)四吡嗪卟嗪锌被用作没有分子内电荷转移的对照组。在 DMF 中,它们的荧光和单线态氧量子产率较高,而在较高浓度的硫酸中,它们的荧光和单线态氧量子产率会因偶氮甲基硝基的质子化而降低。结果表明,所研究化合物的光物理和光化学性质可能受介质 pH 值变化的控制。
表征谱图
-
氢谱1HNMR
-
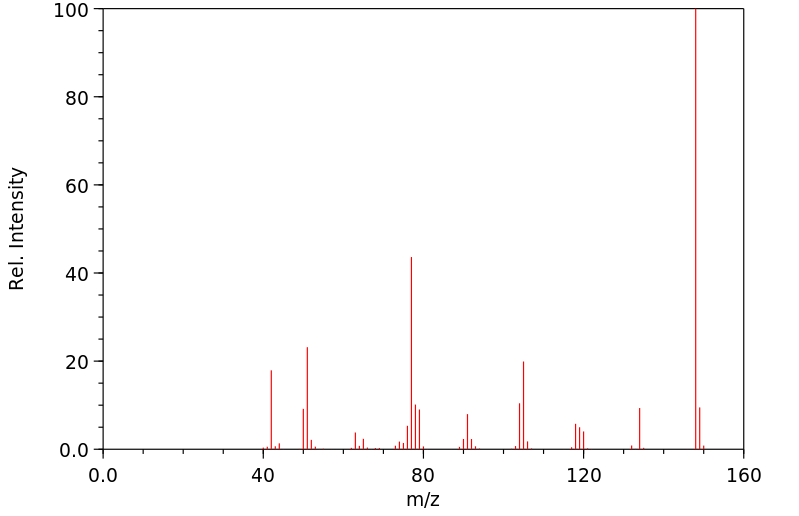
质谱MS
-
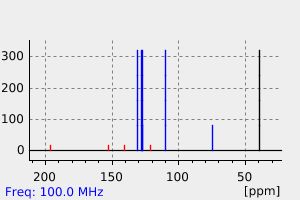
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息