7-oxo-1,3,5-cycloheptatrienecarbonitrile | 934-19-0
中文名称
——
中文别名
——
英文名称
7-oxo-1,3,5-cycloheptatrienecarbonitrile
英文别名
2-cyanotropone;7-oxo-cyclohepta-1,3,5-trienecarbonitrile;7-Oxo-cyclohepta-1,3,5-triencarbonitril;2-Cyan-tropon;7-Oxo-1,3,5-cycloheptatriene-1-carbonitrile;7-oxocyclohepta-1,3,5-triene-1-carbonitrile
CAS
934-19-0
化学式
C8H5NO
mdl
——
分子量
131.134
InChiKey
UZTIHVRSZUIDTL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:40.9
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:参考文献:名称:The Reaction of 3-Carboxy- and 3-Cyanotropolone with Hydrazine摘要:DOI:10.1246/bcsj.37.1292
-
作为产物:描述:7-oxo-1,5-cycloheptadienecarbonitrile 在 三乙胺 、 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 二氯甲烷 为溶剂, 反应 3.0h, 以76%的产率得到7-oxo-1,3,5-cycloheptatrienecarbonitrile参考文献:名称:扩环方案:合成通用的二氢氢萘酮的制备摘要:扩环方案,包括将各种烯醇盐亲核试剂1,2-加成至6-三甲基甲硅烷氧基-2-环己烯-1-酮(1)和NaIO 4促进所得二醇2的氧化开环,然后进行分子内的Knoevenagel缩合,提供了多用途的二氢萘酮6。保持氧化性开环产物3中的Z构型对于扩环策略的成功至关重要。二氢萘酮6已经成熟,可以进行进一步的精制,例如氧化为对苯二酚8以及与Danishefsky's diene 10的Diels-Alder反应,得到多环化合物12。DOI:10.1021/jo802064c
文献信息
-
THE SYNTHESES OF IRON CARBONYL COMPLEXES OF 2-SUBSTITUTED TROPONES作者:Noboru Morita、Toyonobu Asao、Akio Tajiri、Hideo Sotokawa、Masahiro HatanoDOI:10.1246/cl.1985.1879日期:1985.12.52-Substituted tropones reacted with Fe2(CO)9 or with Fe(CO)5 under irradiation to give iron tricarbonyl complexes coordinated at unsubstituted diene moiety. 2-Bromotropone afforded another iron tricarbonyl complex coordinated at substituted diene as a minor product. 2-Methyl-, 2-phenyl-, and 2-tosyloxytropones yielded di-π-allyl type diiron hexacarbonyl complexes. Furthermore, 2-dialkylaminotropones
-
Reactions of Trimethylsilyl Cyanide with Tropone, 2-Phenyltropone, and 2-Methoxytropone. Formations of 2-Cyano-1-hydroxytropylidene Derivatives作者:Katsuhiro Saito、Hisashi KojimaDOI:10.1246/bcsj.58.1918日期:1985.7Reaction of trimethylsilyl cyanide (TMSCN) with tropone and 2-phenyltropone in the presence of a catalytic amount of zinc iodide in dichloromethane afforded silyl ethers of 7-cyano-1,3,5-cycloheptatriene-1-ol (2a) and 2-phenyl-7-cyano-1,3,5-cycloheptatriene-1-ol (2b), respectively. On the other hand, reaction of TMSCN with 2-methoxytropone gave 2-cyanotropone. Upon hydrolysis, 2a, and 2b yielded 2-cyano-1在二氯甲烷中催化量的碘化锌存在下,三甲基氰化甲硅烷 (TMSCN) 与托酮和 2-苯基托酮反应,得到 7-氰基-1,3,5-环庚三烯-1-醇 (2a) 和 2- 的甲硅烷基醚苯基-7-氰基-1,3,5-环庚三烯-1-醇 (2b),分别。另一方面,TMSCN 与 2-甲氧基托酮反应得到 2-氰托酮。水解后,2a 和 2b 生成 2-氰基-1,3,5-环庚三烯-1-醇 (3a) 和 2-氰基-7-苯基-1,3,5-环庚三烯-1-醇的互变异构混合物(3b) 和 2-cyano-7-phenyl-3,5-cycloheptadien-1-one (4b)。在二恶烷中用二氧化硒氧化 3a 和 3b 和 4b 的互变异构混合物,分别得到 2-氰基-7-苯基托酮和 2-氰基-7-苯基托酮。
-
Nozoe et al., Proceedings of the Japan Academy, 1952, vol. 28, p. 488作者:Nozoe et al.DOI:——日期:——
-
SAITO, KATSUHIRO;KOJIMA, HISASHI, BULL. CHEM. SOC. JAP., 1985, 58, N 7, 1918-1921作者:SAITO, KATSUHIRO、KOJIMA, HISASHIDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
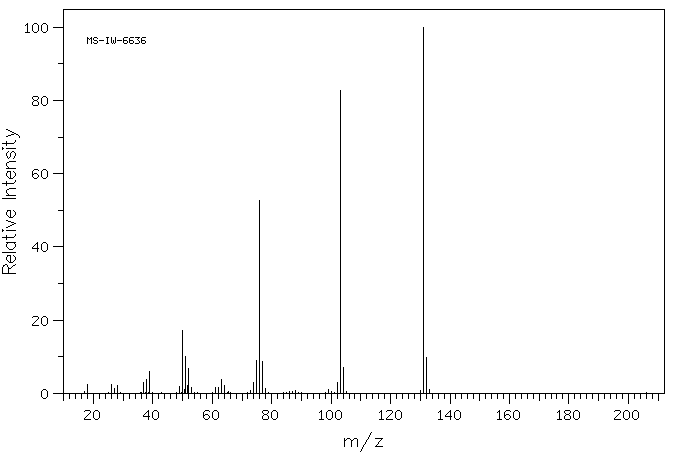
质谱MS
-
碳谱13CNMR
-
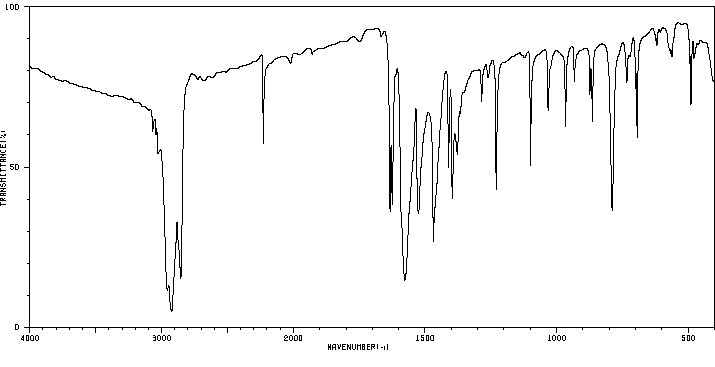
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-