3-Phenyl-octan | 18335-15-4
中文名称
——
中文别名
——
英文名称
3-Phenyl-octan
英文别名
3-phenyloctane;Octane, 3-phenyl-;octan-3-ylbenzene
CAS
18335-15-4
化学式
C14H22
mdl
——
分子量
190.329
InChiKey
XEYWPMIXBVMRLE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1352;1355;1358;1366
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:14
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-辛烷基苯 4-phenyloctane 18335-17-6 C14H22 190.329 辛-1,7-二烯-3-基苯 octa-1,7-dien-3-ylbenzene 112176-11-1 C14H18 186.297 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (1-甲基庚基)苯. 2-phenyloctane 777-22-0 C14H22 190.329
反应信息
-
作为反应物:描述:参考文献:名称:Nasterova, T. N.; Roshchupkina, I. Yu.; Pimerzin, A. A., Journal of applied chemistry of the USSR, 1988, vol. 61, # 7, p. 1445 - 1449摘要:DOI:
-
作为产物:描述:参考文献:名称:Nasterova, T. N.; Roshchupkina, I. Yu.; Pimerzin, A. A., Journal of applied chemistry of the USSR, 1988, vol. 61, # 7, p. 1445 - 1449摘要:DOI:
文献信息
-
Silica-Supported Heteropoly Acid Catalyst for Liquid-Phase Friedel–Crafts Reactions作者:Yusuke Izumi、Noriko Natsume、Harumi Takamine、Ikuyo Tamaoki、Kazuo UrabeDOI:10.1246/bcsj.62.2159日期:1989.7heteropoly acids such as 12-tungstophosphoric, molybdophosphoric, and tungstosilicic acids efficiently catalyzed Friedel–Crafts alkylation and acylation reactions of aromatic hydrocarbons with 1-octene, benzyl chloride and benzoyl chloride in the liquid phase. The catalytic activity of heteropoly acid was generally enhanced when supported on silica. Silica-supported tungstosilicic acid worked as an
-
Adsorption and catalytic properties of sulfated aluminum oxide modified with cobalt ions作者:S. N. Lanin、A. A. Bannykh、E. V. Vlasenko、I. N. Krotova、O. N. Obrezkov、M. I. ShilinaDOI:10.1134/s0036024417010150日期:2017.1The adsorption properties of sulfated aluminum oxide (9% SO 2-4 /γ-Al2O3) and a cobalt-containing composite (0.5%Сo/SO 2-4 /γ-Al2O3) based on it are studied via dynamic sorption. The adsorption isotherms of such test adsorbates as n-hydrocarbons (C6–C8), benzene, ethylbenzene, chloroform, and diethyl ether are measured, and their isosteric heats of adsorption are calculated. It is shown that the surface硫酸化氧化铝的吸附性能(9%SO 2- 4 /γ-Al系2 ö 3)和含钴复合(0.5%Сo/ SO 2- 4 /γ-Al系2 ö 3)的基础上它进行了研究通过动态吸附。测量这些测试吸附物的吸附等温线,如正烃(C 6 –C 8),苯,乙苯,氯仿和二乙醚,并计算其等规吸附热。结果表明,三氧化二铝的表面基本上硫酸化提高了其电子接受性,所以SO的催化活性2- 4 /γ-Al系2 ö 3 在25–120°C的温度下,苯与辛烯1的液相烷基化反应比最初的氧化铝要高一个数量级。可以确定的是,用钴离子对硫酸氧化铝进行了其他改性,从而使该催化剂的活性提高了2-4倍。结果表明,能够强特异性吸附的吸附部位与两个捐赠(芳族化合物,二乙醚化学吸附)和接受硫酸化的γ-Al表面上的分子(氯仿)形式2 ö 3通过钴盐促进。
-
Detailed Characterization of <i>p</i>-Toluenesulfonic Acid Monohydrate as a Convenient, Recoverable, Safe, and Selective Catalyst for Alkylation of the Aromatic Nucleus作者:Mathew P. D. Mahindaratne、Kandatege WimalasenaDOI:10.1021/jo971832r日期:1998.5.1Alkylation of the aromatic nucleus, an important reaction in industry and synthetic organic chemistry, has traditionally been carried out by the well-known Friedel-Crafts reaction employing Lewis acid catalysts such as AlCl3 and BF3 or by using highly reactive organometallic reagents. Although protic acids such as anhydrous HF and concentrated H2SO4 have also been used in the alkylation of the aromatic nucleus, the notoriously corrosive, highly toxic, and hazardous nature of these agents has precluded their common use under ordinary laboratory conditions. Various organic sulfonic acids have, on occasion, been used as catalysts in Friedel-Crafts alkylations, but to our knowledge the chemistry and the scope of these reactions for common laboratory use have never been exploited in detail. In the present study we have characterized commercially available p-toluenesulfonic acid monohydrate (TsOH) as an efficient catalyst for the intermolecular coupling of the aromatic nucleus with activated alkyl halides, alkenes, or tosylates under mild conditions in an open atmosphere. In comparison to conventional Friedel-Crafts catalysts such as AlCl3, BF3, HF, and concentrated H2SO4, the extent of the formation of undesired products from side reactions such as transalkylation, polymerization, etc. was minimal with the TsOH-catalyzed reaction. The ability to recover and reuse the catalyst from the reaction mixtures, minimal generation of environmentally unfriendly waste, high specificity of the reaction, and the low cost of the catalyst are important advantages of the TsOH catalyst over the other conventional Friedel-Crafts catalysts.
-
Selective hydrogenation of substituted dienes catalyzed by an organoyttrium complex作者:Gary A. Molander、John O. HobergDOI:10.1021/jo00038a004日期:1992.6Cp*2YMe(THF) has been developed as an efficient catalyst for the selective reduction of substituted dienes.
-
Olson, Industrial and Engineering Chemistry, 1960, vol. 52, p. 835作者:OlsonDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
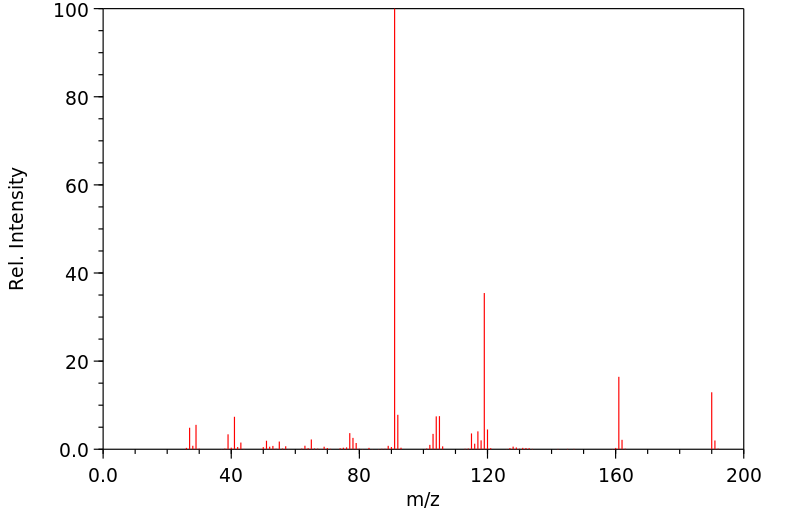
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫