4-叔-戊基苯氧基乙醇 | 6382-07-6
中文名称
4-叔-戊基苯氧基乙醇
中文别名
——
英文名称
2-(4-tert-pentyl-phenoxy)-ethanol
英文别名
(2-Hydroxy-aethyl)-(4-tert.-pentyl-phenyl)-aether;2-Hydroxy-1-(4-tert.-pentyl-phenoxy)-aethan;2-(4-tert-Pentyl-phenoxy)-aethanol;2-[4-(1,1-dimethylpropyl)phenoxy]ethanol;2-[4-(2-methylbutan-2-yl)phenoxy]ethanol
CAS
6382-07-6
化学式
C13H20O2
mdl
——
分子量
208.301
InChiKey
BXXDXUTVAFRBKC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:314.8±25.0 °C(Predicted)
-
密度:0.988±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.54
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2909499000
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对叔戊基苯酚 4-t-amylphenol 80-46-6 C11H16O 164.247
反应信息
-
作为反应物:描述:参考文献:名称:Halomethyl ethers of nuclear alkylated monoaryl glycol ethers摘要:公开号:US02554441A1
-
作为产物:描述:参考文献:名称:US2158959摘要:公开号:
文献信息
-
Controlled release solid dispersions of carvedilol申请人:Egalet A/S公开号:EP1929998A2公开(公告)日:2008-06-11A controlled release pharmaceutical composition for oral use comprising a solid dispersion of i) carvedilol, which at least partially is in an amorphous form, ii) a pharmaceutically acceptable polymer that has plasticizing properties and which has a melting point or melting interval of a temperature of at the most 200 °C, and the composition being designed to release carvedilol with a substantially zero order release, The polymer is typically a polyethylene glycol and/or a polyethylene oxide having a molecular weight of at least about 20,000 in crystalline and/or amorphous form or a mixture such polymers, and the active substance is typically carvedilol.
-
Matrix compositions for controlled delivery of drug substances申请人:Egalet A/S公开号:EP1974726A2公开(公告)日:2008-10-01A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and/or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200 °C, the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides having a molecular weight of at least about 20,000 in crystalline and/or amorphous form or a mixture such polymers, and the second polymer being selected from block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid) - b- ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid) - g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide - polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and/or diagnostically active substance, wherein the concentration of the second polymer in the matrix composition is from about 5 to about 90% w/w, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, the coating comprising i) a first cellulose derivative which has thermoplastic properties and which is substantially insoluble in the aqueous medium in which the composition is to be used, and at least one of ii) a second cellulose derivative which is soluble or dispersible in water, iii) a plasticizer, and iv) a filler, wherein the active substance is released with a substantially zero order release.一种新型药用基质组合物。基质组合物的设计使其特别适用于需要提高生物利用率的情况和/或使用微溶或不溶活性物质的情况。因此,一种口服控释药物组合物以包衣基质组合物的形式提供,该基质组合物包括 i) 第一种聚合物和第二种聚合物的混合物,这两种聚合物具有塑化特性,其熔点或熔 化间隔温度最高可达 200 °C、 第一种聚合物选自分子量至少约为 20,000 的结晶和/或无定形形式的聚乙二醇和聚乙 烯氧化物或此类聚合物的混合物,以及 第二种聚合物选自环氧乙烷和环氧丙烷的嵌段共聚物,包括聚乙二醇-b-(DL-乳酸-共羟基乙酸)-b-乙二醇(PEG-PLGA PEG)、聚((DL-乳酸-共羟基乙酸)-g-乙二醇)(PLGA-g-PEG)、聚氧化酰胺和聚环氧乙烷-聚环氧丙烷(PEO-PPO)、 治疗、预防和/或诊断活性物质、 其中基质组合物中第二种聚合物的浓度约为 5%至 90%(重量百分比)、 基质组合物上有一层涂层,该涂层至少有一个开口露出基质的一个表面,涂层包括 i) 第一种纤维素衍生物,它具有热塑性,并且基本上不溶于使用该组合物的水介质中、 以及以下至少一种 可溶于或分散于水的第二种纤维素衍生物、 增塑剂 iv) 填充剂、 其中活性物质的释放基本为零阶释放。
-
Morphine controlled release system申请人:Egalet A/S公开号:EP2301526A2公开(公告)日:2011-03-30A pharmaceutical composition for controlled release of at least one opioid into an aqueous medium by erosion of at least one surface of the composition, the composition comprising i) a matrix composition comprising a) a polymer or a mixture of polymers, wherein the polymer comprises a polyethylene glycol, a polyethylene oxide and/or a block copolymer of ethylene oxide and propylene oxide, b) an opioid as an active substance and, optionally, c) one or more pharmaceutically acceptable excipients, and ii) a coating having at least one opening exposing at the one surface of said matrix, the coating comprising a polymer selected from polyamide, polyethylene, polyethylene terephthalate, polypropylenem polyurethane, polyvinyl acetate, polyvinyl chloride, silicone rubber, latex, polyhydroxybutyrate, polyhydroxyvalerate, teflon, polylactic acid or polyglycolic acid and copolymers thereof, copolymers such as ethylene vinyl acetate (EVA), styrene-butadienestyrene (SBS) and styrene-isoprene-styrene (SIS), wherein the matrix composition has a conus-like shape so that the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid - when tested in a Dissolution Test in accordance with the USP 24, NF 19, (711), Dissolution, apparatus 2 equipped with a paddle; with or without application of sinkers - results in a zero order release of at least 80% of the opioid contained in the composition, and about 75% w/w of the opioid is released from the composition within 4-10 hours.一种药物组合物,用于通过侵蚀组合物的至少一个表面将至少一种阿片类药物控 制释放到水介质中,该组合物包括 i) 基质组合物,包括 a) 聚合物或聚合物混合物,其中聚合物包括聚乙二醇、聚环氧乙烷和/或环氧乙烷与环氧丙烷的嵌段共聚物;b) 作为活性物质的阿片类药物;以及 c) 一种或多种药学上可接受的赋形剂,以及 硅橡胶、乳胶、聚羟基丁酸酯、聚羟基戊酸酯、聚四氟乙烯、聚乳酸或聚乙醇酸及其共聚物,以及乙烯-醋酸乙烯(EVA)、苯乙烯-丁二烯-苯乙烯(SBS)和苯乙烯-异戊二烯-苯乙烯(SIS)等共聚物、 其中,基质组合物具有圆锥状形状,这样至少在基质组合物的初始侵蚀期间,暴露于水介质的表面积会增加,并且 阿片类药物的溶解--当按照美国药典 24,NF 19,(711),溶解,配备桨的仪器 2 进行溶解测试时;无论是否使用沉降片--导致组合物中所含阿片类药物至少 80% 的零阶释放,约 75% w/w 的阿片类药物在 4-10 小时内从组合物中释放。
-
Zero-order modified release solid dosage forms申请人:Mallinckrodt LLC公开号:EP2457562A1公开(公告)日:2012-05-30The invention comprises a solid dosage form for delivery of water soluble pharmaceutical agents. The solid dosage form comprises a matrix core containing the pharmaceutical agent and a hydrophobic material; a modified release coating containing a hydrophilic pore-forming agent and a hydrophobic polymer; and a water-soluble barrier coating between the modified release coating and the matrix core. The dosage form exhibits a zero-order release profile upon dissolution.
-
Morphine polymer release system申请人:——公开号:US20040253310A1公开(公告)日:2004-12-16A pharmaceutical composition for controlled release of an active substance. The active substance is released into an aqueous medium by erosion of at least one surface of the composition. The composition comprises i) a matrix comprising a) polymer or a mixture of polymers, b) an active substance and, optionally, c) one or more pharmaceutically acceptable excipients, and ii) a coating. Zero order release is desirable. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof. The coating comprises a first cellulose derivative which is substantially insoluble in the aqueous medium and at least one of a) a second cellulose derivative which is soluble or dispersible in water, b) a plasticizer, and, d) a filler.
表征谱图
-
氢谱1HNMR
-
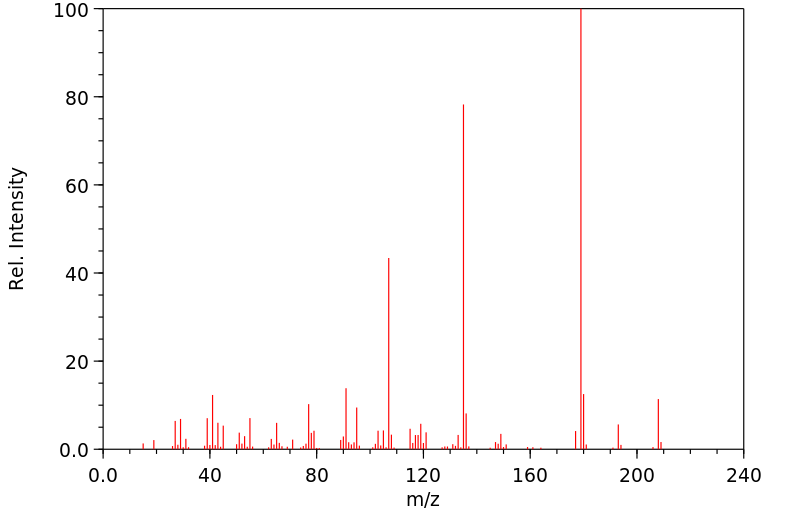
质谱MS
-
碳谱13CNMR
-
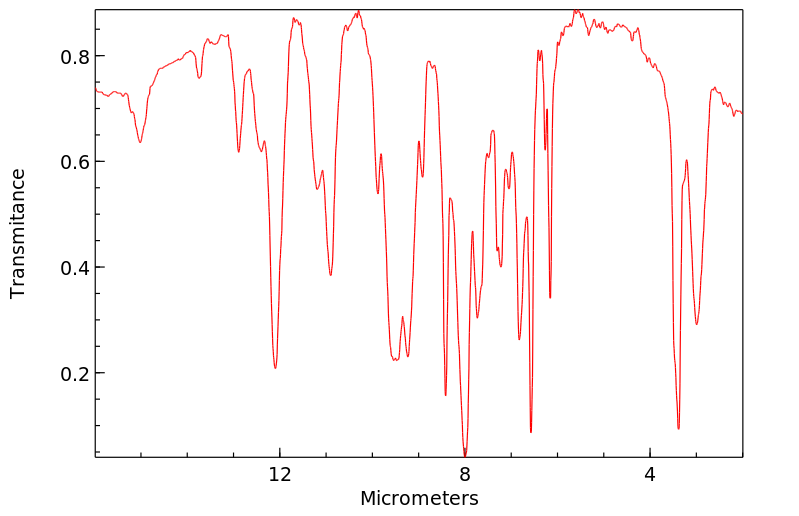
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫