4-氨基-2,3-二甲基-苯酚盐酸盐(1:1) | 94276-04-7
中文名称
4-氨基-2,3-二甲基-苯酚盐酸盐(1:1)
中文别名
肼碳杂氧杂脒,N-甲基-1-[2-(4-甲基-1-哌啶基)乙基]-
英文名称
4-amino-2,3-dimethyl-1-hydroxybenzene hydrochloride
英文别名
4-amino-2,3-dimethylphenol, hydrochloride;4-Amino-2,3-dimethylphenol hydrochloride;4-Amino-2,3-xylenol hydrochloride;4-amino-2,3-dimethylphenol;hydrochloride
CAS
94276-04-7
化学式
C8H11NO*ClH
mdl
——
分子量
173.642
InChiKey
GXLRCJUNACHBAX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.01
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:46.2
-
氢给体数:3
-
氢受体数:2
SDS
反应信息
-
作为反应物:描述:4-氨基-2,3-二甲基-苯酚盐酸盐(1:1) 在 咪唑 、 盐酸 作用下, 以 甲醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 7.0h, 生成 N-(4-hydroxy-2,3-dimethyl-phenyl)acetamide参考文献:名称:The fischer indolisation reaction and the synthesis of dihydroindenoindoles摘要:The Fischer reaction between indanones and certain alkoxyarylhydrazines fails; the indanones are returned unreacted and the arylhydrazines are converted into the corresponding alkoxy-2-chloroarylamines and other products. A new N-amination route to arylhydrazines from the arylamines has been developed and it has been demonstrated that problems with the indolisation of alkoxyarylhydrazones can be circumvented by ring closures of their O-tosylated analogues. Some results using the Lepke synthesis of indoles are recorded.DOI:10.1016/s0040-4020(01)81911-6
文献信息
-
2-(hydroxy-2-alkylphenylamino)-oxazolines and thiazolines as申请人:Allergan, Inc.公开号:US05066664A1公开(公告)日:1991-11-19Compounds of the formula ##STR1## where X is O or S; R.sub.1 is lower alkyl having 1 to 6 carbons; R.sub.2 is H, lower alkyl having 1 to 6 carbons; or OR.sub.2.sup.* where R.sub.2.sup.* is lower alkyl having 1 to 6 carbons; Y is O, S or NH; and R.sub.3 is H, lower alkyl having 1 to six carbons, or C(O)R.sub.3.sup.* where R.sub.3.sup.* is lower alkyl having 1 to 5 carbons, with the proviso that the YR.sub.3 group is not disposed in the ortho position on the benzene nucleus relative to the 2-amino group of the oxazoline or thiazoline heterocycle, are active in maintaining or reducing intraocular pressure in the mammalian eye, and are active as vasoconstrictors and capable of reducing or controlling intraocular bleeding of the mammalian eye.
-
Quinoline derivatives and quinazoline derivatives申请人:KIRIN BEER KABUSHIKI KAISHA公开号:US20040209905A1公开(公告)日:2004-10-21An object of the present invention is to provide compounds which have antitumor activity and do not change cytomorphosis. Disclosed are compounds represented by formula (I) and a pharmaceutically acceptable salts and solvates thereof and pharmaceutical compositions comprising said compounds: 1 wherein X and Z each independently represent CH or N; R 1 to R 3 represent H, substituted alkoxy, unsubstituted alkoxy or the like; R 4 represents H; R 5 to R 8 represent H, halogen, alkyl, alkoxy, alkylthio, nitro, or amino, provided that R 5 to R 8 do not simultaneously represent H; R 9 and R 10 represent H, alkyl, or alkylcarbonyl; and R 11 represents alkyl, alkenyl, alkynyl, or aralkyl.
-
Derivatives of N-((quinolinyl)oxy)-phenyl)-urea and N-((quinazolinyl)oxy)-phenyl)-urea with antitumor activity申请人:KIRIN BEER KABUSHIKI KAISHA公开号:EP1384712A1公开(公告)日:2004-01-28An object of the present invention is to provide compounds which have antitumor activity and do not change cytomorphosis. Disclosed are compounds represented by formula (I) and a pharmaceutically acceptable salts and solvates thereof and pharmaceutical compositions comprising said compounds: wherein X and Z each independently represent CH or N; R1 to R3 represent H, substituted alkoxy, unsubstituted alkoxy or the like; R4 represents H; R5 to R8 represent H, halogen, alkyl, alkoxy, alkylthio, nitro, or amino, provided that R5 to R8 do not simultaneously represent H; R9 and R10 represent H, alkyl, or alkylcarbonyl; and R11 represents alkyl, alkenyl, alkynyl, or aralkyl.
-
QUINOLINE DERIVATIVES AND QUINAZOLINE DERIVATIVES申请人:KIRIN BEER KABUSHIKI KAISHA公开号:EP1153920B1公开(公告)日:2003-10-29
-
US6797823B1申请人:——公开号:US6797823B1公开(公告)日:2004-09-28
表征谱图
-
氢谱1HNMR
-
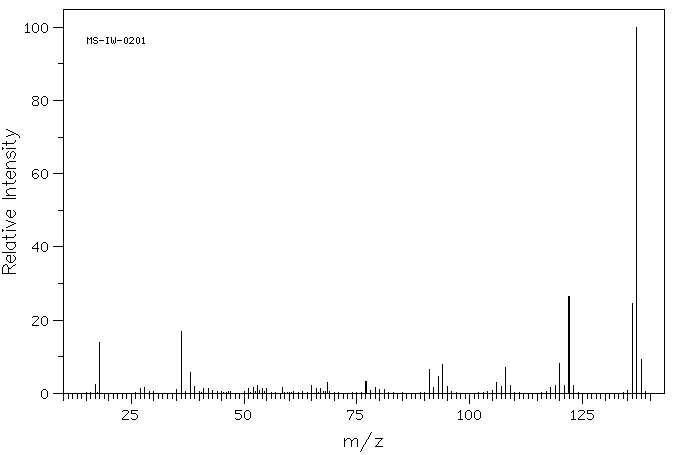
质谱MS
-
碳谱13CNMR
-
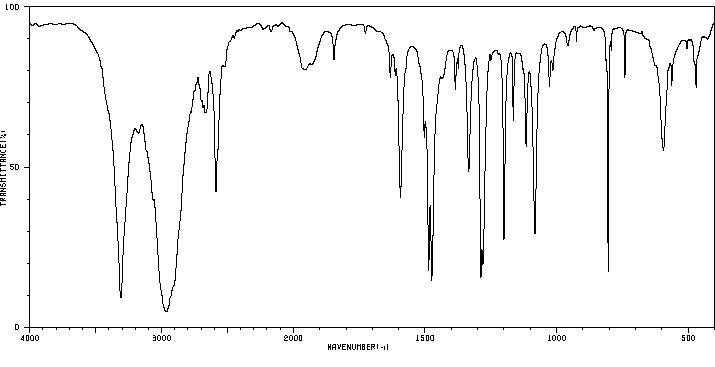
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚