3-amino-7-ethyl-2,5,6,8-tetrahydroxy-1,4-dihydronaphthlene-1,4-dione | 866365-79-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.47
-
重原子数:19.0
-
可旋转键数:1.0
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:141.08
-
氢给体数:5.0
-
氢受体数:7.0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-amino-7-ethyl-5,8-dihydroxy-2,6-dimethoxy-1,4-naphthoquinone 866229-66-5 C14H15NO6 293.276 —— 7-ethyl-2,5,6,8-tetrahydroxy-3-nitronaphthalene-1,4-dione 1292276-30-2 C12H9NO8 295.205 —— echinochrome A 1471-96-1 C12H10O7 266.207 —— 3-chloro-7-ethyl-5,6,8-trihydroxy-2-methoxy-1,4-naphthoquinone 880154-58-5 C13H11ClO6 298.68 —— 2-azido-3-chloro-7-ethyl-5,6,8-trihydroxynaphthalene-1,4-dione 880154-54-1 C12H8ClN3O5 309.666 —— 5,8-dihydroxy-2,6-dimethoxy-3-chloro-7-ethyl-1,4-naphthoquinone 475084-15-2 C14H13ClO6 312.707 —— 5,8-Dihydroxy-2,3-dichloro-6-ethyl-7-ethoxy-1,4-naphthoquinone 168140-17-8 C14H12Cl2O5 331.152 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-amino-7-ethyl-5,8-dihydroxy-2,6-dimethoxy-1,4-naphthoquinone 866229-66-5 C14H15NO6 293.276 —— echinochrome A 1471-96-1 C12H10O7 266.207
反应信息
-
作为反应物:描述:3-amino-7-ethyl-2,5,6,8-tetrahydroxy-1,4-dihydronaphthlene-1,4-dione 在 甲酸 、 硫酸 、 二甲基亚砜 作用下, 以 水 为溶剂, 反应 0.17h, 以81%的产率得到echinochrome A参考文献:名称:DMSO介导的3-氨基-2-羟基萘他沙林向天然2,3-二羟基萘他沙林及其相关化合物的转化摘要:已经开发了用于合成天然存在的和相关的羟基化萘萘沙林的通用且方便的方法。该方案涉及在酸性条件下将DMSO介导的3-氨基-2-羟基萘萘氧化为2,3-二羟基萘萘。基于实验观察,提出了合理的反应机理。DOI:10.1016/j.tetlet.2016.06.056
-
作为产物:描述:2-azido-3-chloro-7-ethyl-5,6,8-trihydroxynaphthalene-1,4-dione 在 sodium azide 、 氢溴酸 、 potassium carbonate 、 溶剂黄146 作用下, 以 乙醚 、 二甲基亚砜 为溶剂, 反应 6.0h, 生成 3-amino-7-ethyl-2,5,6,8-tetrahydroxy-1,4-dihydronaphthlene-1,4-dione参考文献:名称:Regiospecificity in the reaction of 2,3-dichloronaphthazarins with azide anions. Synthesis of echinamine A—a metabolite produced by the sea urchin Scaphechinus mirabilis摘要:It was found that 6-hydroxy- and 6-alkoxy-2,3-dichloronaphthazarins react smoothly with sodium azide in methanol to produce the corresponding 2-azido derivatives as single regioisomers. We have explored the utility of this reaction for the synthesis of echinamine A (3-amino-7-ethyl-2,5,6,8-tetrahydroxy-1,4-naphthoquinone)-the first marine aminated hydroxynaphthazarin, a metabolite of the sea urchin Scaphechinus mirabilis (Agassiz). (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2005.12.109
文献信息
-
Reaction of Dichloronaphthazarins with Sodium Nitrite as a Route to Natural Pigments Echinamines A and B and Related Aminonaphthazarins作者:Nikita Polonik、Sergey Polonik、Vladimir Denisenko、Olga MoiseenkoDOI:10.1055/s-0030-1260229日期:2011.10A series of 6,7-disubstituted 2-hydroxy-3-nitronaphthazarins were prepared by treatment of 2,3-dichloronaphthazarins with sodium nitrite. Acid-catalyzed hydrolysis of a mixture of two isomeric 6(7)-ethoxy-7(6)-ethyl-substituted 2-hydroxy-3-nitronaphthazarins followed by chromatographic separation led to the individually precursors of echinamines A and B. Further reduction of nitroquinones using various reducing agents gave echinamines and related 3-amino-2-hydroxynaphthazarins in good yields.
-
Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1作者:Natalia P. Mishchenko、Natalia V. Krylova、Olga V. Iunikhina、Elena A. Vasileva、Galina N. Likhatskaya、Evgeny A. Pislyagin、Darya V. Tarbeeva、Pavel S. Dmitrenok、Sergey A. FedoreyevDOI:10.3390/md18110550日期:——
Herpes simplex virus type 1 (HSV-1) is one of the most prevalent pathogens worldwide requiring the search for new candidates for the creation of antiherpetic drugs. The ability of sea urchin spinochromes—echinochrome A (EchA) and its aminated analogues, echinamines A (EamA) and B (EamB)—to inhibit different stages of HSV-1 infection in Vero cells and to reduce the virus-induced production of reactive oxygen species (ROS) was studied. We found that spinochromes exhibited maximum antiviral activity when HSV-1 was pretreated with these compounds, which indicated the direct effect of spinochromes on HSV-1 particles. EamB and EamA both showed the highest virucidal activity by inhibiting the HSV-1 plaque formation, with a selectivity index (SI) of 80.6 and 50.3, respectively, and a reduction in HSV-1 attachment to cells (SI of 8.5 and 5.8, respectively). EamA and EamB considerably suppressed the early induction of ROS due to the virus infection. The ability of the tested compounds to directly bind to the surface glycoprotein, gD, of HSV-1 was established in silico. The dock score of EchA, EamA, and EamB was −4.75, −5.09, and −5.19 kcal/mol, respectively, which correlated with the SI of the virucidal action of these compounds and explained their ability to suppress the attachment and penetration of the virus into the cells.
单纯疱疹病毒1型(HSV-1)是全球最常见的病原体之一,需要寻找新的候选药物来创造抗单纯疱疹药物。研究了海胆刺色素 - 刺胭脂A(EchA)及其氨基化类似物刺胭脂胺A(EamA)和B(EamB)抑制Vero细胞中HSV-1感染的不同阶段以及减少病毒诱导的活性氧(ROS)产生的能力。我们发现,当HSV-1经过这些化合物的预处理时,刺色素表现出最大的抗病毒活性,这表明刺色素对HSV-1颗粒具有直接影响。EamB和EamA通过抑制HSV-1斑疹形成表现出最高的病毒灭活活性,分别具有选择性指数(SI)为80.6和50.3,并减少HSV-1附着于细胞上(分别为8.5和5.8的SI)。EamA和EamB显著抑制了病毒感染早期ROS的诱导。通过体外模拟,建立了被测试化合物直接结合到HSV-1表面糖蛋白gD的能力。EchA,EamA和EamB的对接得分分别为-4.75,-5.09和-5.19 kcal / mol,与这些化合物的灭活作用的SI相关,并解释了它们抑制病毒附着和穿透细胞的能力。 -
Amination of 2-hydroxy- and 2,3-dihydroxynaphthazarins. Synthesis of echinamines A and B, metabolites produced by the sand dollar Scaphechinus mirabilis作者:G. I. Mel’man (Sopel’nyak)、N. P. Mishchenko、V. A. Denisenko、D. V. Berdyshev、V. P. Glazunov、V. F. AnufrievDOI:10.1134/s1070428009010060日期:2009.12-Hydroxynaphthazarins reacted with aqueous ammonia at the C(1)=O carbonyl group, following the addition-elimination pattern with formation of 8-aminojuglone derivatives. Reactions of 2,3-dihydroxynaphthazarins with the same reagent involved the C(2)=O carbonyl group of the corresponding 1,2-naphthoquinoid tautomer to produce 2-aminonaphthazarin derivatives. 7-Ethyl-2,3,6-trihydroxynaphthazarin (echinochrome) reacted with aqueous ammonia to give 3(2)-amino-7-ethyl-2(3),6-dihydroxynaphthazarins (echinamines A and B) that are metabolites recently isolated from the sand dollar Scaphechinus mirabilis.
-
Synthesis of Echinamines A and B, the First Aminated Hydroxynaphthazarins Produced by the Sea Urchin <i>Scaphechinus </i><i>m</i><i>irabilis</i> and Its Analogues作者:Nataly D. Pokhilo、Maria I. Shuvalova、Maxim V. Lebedko、Galina I. Sopelnyak、Alla Ya. Yakubovskaya、Natalia P. Mischenko、Sergey A. Fedoreyev、Victor Ph. AnufrievDOI:10.1021/np0502185日期:2006.8.1The first total synthesis of two marine aminated hydroxynaphthazarins, echinamines A (3-amino-7-ethyl- 2,5,6,8-tetrahydroxy-1,4- naphthoquinone) and B (2-amino-7-ethyl- 3,5,6,8-tetrahydroxy-1,4- naphthoquinone), produced by the sea urchin Scaphechinus mirabilis is described. This was achieved from 1,2,4-triacetoxybenzene (13) through a sequence involving double Fries rearrangement of 13, reduction of 3,5-diacetyl-1,2,4-trihydroxybenzene (14), methylation of 3,5-diethyl-1,2,4- trihydroxybenzene (15), simultaneous double acylation of 3,5-diethyl-1,2,4- trimethoxybenzene ( 16) with a dichloromaleic anhydride-ethyl radical elimination process, methylation of 6,7-dichloro-3-ethyl-2-hydroxynaphthazarin (17), nucleophilic substitution of a chlorine atom by the methoxy group in 6,7-dichloro-3-ethyl-2-methoxynaphthazarin (18), introduction of an amino group via direct substitution of a chlorine atom in 7-chloro-3-ethyl-2,6- dimethoxy- (11) and 7-chloro-2-ethyl-3,6-dimethoxynaphthazarins (12) by an azido group, and functional group deprotection. The synthesis of amino analogues of spinazarin and spinochrome D is also described.
-
Echinamines A and B, First Aminated Hydroxynaphthazarins from the Sea Urchin <i>Scaphechinus </i><i>m</i><i>irabilis</i>作者:Natalia P. Mischenko、Sergey A. Fedoreyev、Nataly D. Pokhilo、Victor Ph. Anufriev、Vladimir A. Denisenko、Valery P. GlazunovDOI:10.1021/np049585r日期:2005.9.1Two new spinochromes, echinamines A (1) and B (2), were isolated from the sea urchin Scaphechinus mirabilis. Compounds 1 and 2 represent the first examples of natural polyhydroxynaphthazarins with a primary amine group. The structures of 1 and 2 were established by analysis of spectroscopic data and synthesis of their dimethyl ethers.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
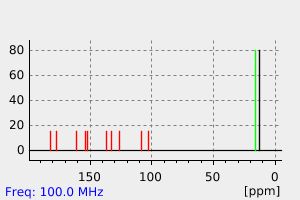
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息