atropine | 51-55-8
中文名称
——
中文别名
——
英文名称
atropine
英文别名
Hyoscyamin;(8-methyl-8-azabicyclo[3.2.1]octan-3-yl) (2R)-3-hydroxy-2-phenylpropanoate
CAS
51-55-8;101-31-5;13269-35-7;16175-85-2;83454-31-3;98302-33-1;131432-21-8
化学式
C17H23NO3
mdl
——
分子量
289.375
InChiKey
RKUNBYITZUJHSG-AUSYRVNMSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:108,5°C
-
比旋光度:D20 -21.0° (alc)
-
沸点:431.53°C (rough estimate)
-
密度:1.0470 (rough estimate)
-
溶解度:DMSO:79.0(最大浓度 mg/mL);273.0(最大浓度 mM)乙醇:58.0(最大浓度 mg/mL);200.43(最大浓度 mM)
-
LogP:1.380 (est)
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:21
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.59
-
拓扑面积:49.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险品标志:T+
-
安全说明:S25,S26,S36,S45
-
危险类别码:R26/28
-
WGK Germany:3
-
海关编码:2939999090
-
RTECS号:CK0700000
-
危险品运输编号:1544
SDS
制备方法与用途
功效作用
体外研究
莨菪碱广泛存在于多种重要中草药中,包括颠茄、北洋金花和曼陀罗。它具有止痛与解痉的作用,对于坐骨神经痛有较好的疗效,并且有时也被用于治疗癫痫和晕船。
生物活性Hyoscyamine(天仙子胺)是一种AChR抑制剂,其IC50值为7.5 nM。
| Target | Value |
|---|---|
| AChR | 7.5 nM |
在稳态动力学测量中,L-hyoscyamine使TPase活性的转化数从0.19 min-¹增加到2.11 min-¹。在CHO细胞中,Hyoscyamine能防止兴奋剂诱导的cAMP产生,EC50值为7.8 nM。S-(-)-hyoscyamine可使小鼠心脏(心房和心室)膜中forskolin刺激产生的环AMP合成增加24%。
体内研究在清醒大鼠体内,L-hyoscyamine (20 mg/kg) 可将移行性肌电复合波(MMC)的循环周期从17.6分钟延长至29.0分钟。
化学性质Hyoscyamine是一种无臭、苦辣味的白色结晶性粉末。易发生消旋,其水溶液呈碱性。熔点为108.5℃,比旋光度[α]20D-21°(乙醇)。该物质极易溶于乙醇和稀酸,在氯仿中的溶解度为1:1,在水中可溶(1:280)、在乙醚中易溶(1:69),在苯中亦可溶(1:159)。
用途Hyoscyamine主要用于生化研究、抗胆碱药及检测金的试剂,也可用于含量测定、鉴定和药理实验等。它通过从颠茄浸膏中提取精制获得。
反应信息
-
作为反应物:参考文献:名称:Differential analgesic activity of the enantiomers of atropine derivatives does not correlate with their muscarinic subtype selectivity摘要:The enantiomers of several tropic and p-substituted tropic acid esters related to atropine obtained by esterification under non-racemizing conditions after resolution of the corresponding racemic acids [(+)- and (-)-18, (+)- and (-)-19] are reported. They were tested in vitro on muscarinic subtype receptors and in vivo for their analgesic activity on mice. As in the case of the lead compound, R-(+)-hyoscyamine, these substances show enantioselectivity in analgesic tests, the eutomers being the R-(+) or R-(+)-p-substituted tropic acid derivatives. However, this property, which is a consequence of increased central release of ACh, seems unrelated to muscarinic subtype selectivity insofar as the compounds are unable to discriminate muscarinic subtype receptors. A possible explanation of these results which does not involve subtype selectivity is proposed, based on the recently developed concept of inverse agonism.DOI:10.1016/s0223-5234(97)83285-0
-
作为产物:参考文献:名称:Differential analgesic activity of the enantiomers of atropine derivatives does not correlate with their muscarinic subtype selectivity摘要:The enantiomers of several tropic and p-substituted tropic acid esters related to atropine obtained by esterification under non-racemizing conditions after resolution of the corresponding racemic acids [(+)- and (-)-18, (+)- and (-)-19] are reported. They were tested in vitro on muscarinic subtype receptors and in vivo for their analgesic activity on mice. As in the case of the lead compound, R-(+)-hyoscyamine, these substances show enantioselectivity in analgesic tests, the eutomers being the R-(+) or R-(+)-p-substituted tropic acid derivatives. However, this property, which is a consequence of increased central release of ACh, seems unrelated to muscarinic subtype selectivity insofar as the compounds are unable to discriminate muscarinic subtype receptors. A possible explanation of these results which does not involve subtype selectivity is proposed, based on the recently developed concept of inverse agonism.DOI:10.1016/s0223-5234(97)83285-0
表征谱图
-
氢谱1HNMR
-
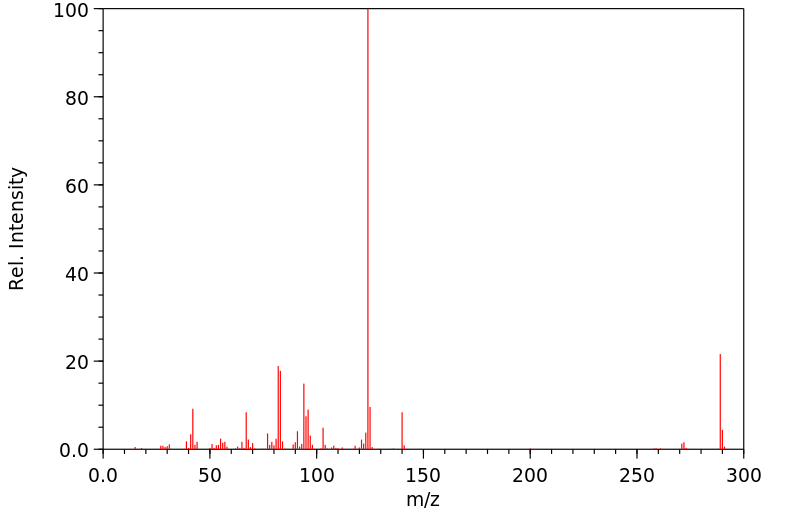
质谱MS
-
碳谱13CNMR
-
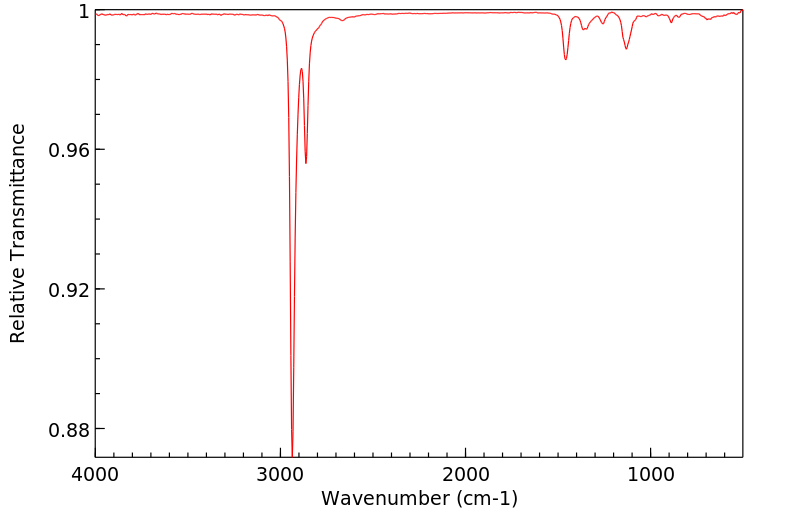
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马拉维若
降莨菪鹼
阿托美品
阿托品硫酸盐
阿托品杂质6
阿托品EP杂质E
辛托溴铵
西克托溴铵
莨菪鹼鹽酸鹽
莨菪碱
莨菪烷
苯酰胺,2-乙氧基-N-[[(8-甲基-8-氮杂二环[3.2.1]辛-3-基)氨基]羰基]-,内-
苯酰胺,2-丁氧基-N-[[(8-甲基-8-氮杂二环[3.2.1]辛-3-基)氨基]羰基]-,内-
苯甲胺,3,5-二氯-N-甲基-
苯甲基2-乙酰氨基-3,6-DI-O-苯甲酰-2-脱氧-α-D-吡喃半乳糖苷
苯基(1R)-3-(4-碘苯基)-8-甲基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
芽子碱盐酸盐
芽子碱甲酯
芽子碱甲基酯盐酸盐
芽子碱
碘氟潘
硫酸莨菪碱水合物
硫酸茛菪素
盐酸去甲托品
白曼陀罗碱
甲硫托铵
甲基8-甲基-3-苯基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R,2S,3S,5S)-3-(4-氯苯基)-8-[(Z)-3-碘丙-2-烯基]-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R,2S,3S,5S)-3-(4-氟苯基)-8-甲基-8-氮杂双环[3.2.1]辛烷-2-甲酸酯
甲基(1R,2S,3S,5S)-3-(4-氟苯基)-8-丙-2-烯基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R)-3-(4-氯苯基)-8-甲基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
甲基(1R)-3-(4-氟苯基)-8-丙基-8-氮杂双环[3.2.1]辛烷-2-羧酸酯
瑞他莫林杂质7
瑞他莫林中间体
环戊烯,1,5-二甲基-3,4-二(亚甲基)-2-(1-甲基乙基)-(9CI)
环己醇并,2-[(4-乙基苯基)氨基]-,(1R,2R)-rel-
特索芬辛
泽泊思定
氢溴酸天仙子胺
氢溴酸天仙子胺
暂无
斯泰因N1
托品醇异丁酸酯
托品醇壬酸酯
托品醇-3-黄酸酯
托品醇
托品酮
托品巴酯
托品-3-硫醇
打碗花精A5