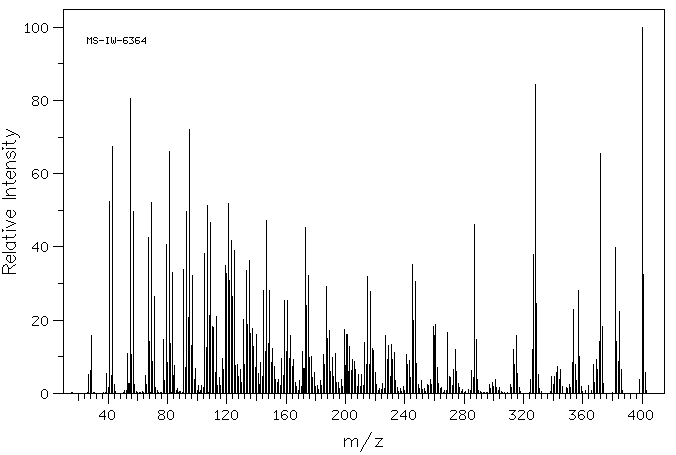
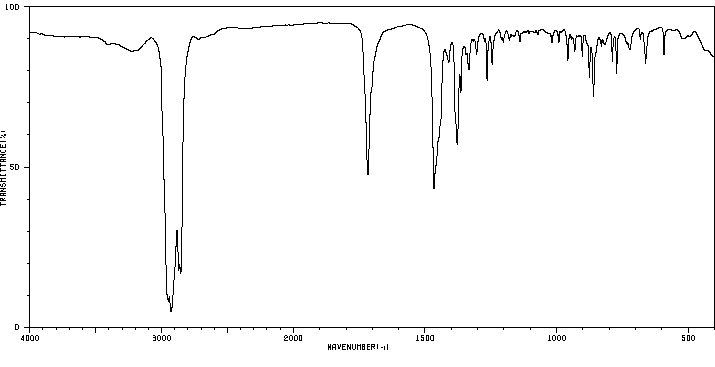
4β,5-epoxy-5β-cholest-3-one | 1975-34-4
分子结构分类
中文名称
——
中文别名
——
英文名称
4β,5-epoxy-5β-cholest-3-one
英文别名
4,5-epoxy-5β-cholestan-3-one;(1S,2R,6R,8R,11S,12S,15R,16R)-2,16-dimethyl-15-[(2R)-6-methylheptan-2-yl]-7-oxapentacyclo[9.7.0.02,8.06,8.012,16]octadecan-5-one
CAS
1975-34-4
化学式
C27H44O2
mdl
——
分子量
400.645
InChiKey
DNHVTVKDKYLDFF-WZHAGKRUSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:116-117 °C
-
沸点:491.5±28.0 °C(Predicted)
-
密度:1.09 g/cm3
计算性质
-
辛醇/水分配系数(LogP):8
-
重原子数:29
-
可旋转键数:5
-
环数:5.0
-
sp3杂化的碳原子比例:0.96
-
拓扑面积:29.6
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:4β,5-epoxy-5β-cholest-3-one 在 吡啶 、 sodium azide 作用下, 生成 1-Acetoxy-4-methyl-19-nor-Δ1,3,5(10)-cholestatrien参考文献:名称:Ahmad, M. S.; Alam, Zafar, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1988, vol. 27, # 1-12, p. 336 - 338摘要:DOI:
-
作为产物:参考文献:名称:Peroxide oxidation of Δ4-3-ketosteroids摘要:Δ4-3-酮类固醇在叔丁基过氧化氢存在下,在氢氧化锂存在下处理,会特异性地形成相应的4β,5β环氧化物,并且收率较高。这种反应的特异性可以通过已接受的氢过氧化物环氧化Δ4-3-酮类固醇的机制来解释。使用水过氧化钠作为氧化剂会导致相应的Δ4-3,6-二酮的生成。对于这种反应提出了一个机制,其中关键步骤是由试剂的作用在原位产生的相应的去共轭Δ5-3-酮类固醇的自氧化。氧化锂不会氧化雄烯-4-烯-3,17-二酮的C-6位,但会产生收率较低的4,5环氧化物,同时伴随着一个A-诺尔-3,5-去氧基酸。DOI:10.1139/v82-268
文献信息
-
Highly efficient epoxidation of unsaturated steroids using magnesium bis(monoperoxyphthalate) hexahydrate作者:João F.S. Carvalho、M. Manuel Cruz Silva、M. Luisa Sá e MeloDOI:10.1016/j.tet.2009.01.100日期:2009.4Fast generation of epoxides from the corresponding homoallylic and allylic steroidal olefins was developed by using magnesium bis(monoperoxyphthalate) hexahydrate (MMPP) as oxidant suspended in acetonitrile (CH3CN) at reflux temperature. The protocol involves the use of a safe readily available oxidant along with an easy work-up, which renders the process very efficient. Selective 4,5- and 5,6-epoxidations
-
Diastereoselective epoxidation of olefins by organo sulfonic peracids, II作者:R. Kluge、M. Schulz、S. LiebschDOI:10.1016/0040-4020(95)01128-5日期:1996.2have investigated the behaviour of sulfonic peracids 2in situ generated towards olefins 7a,7b,9,11,14,16,18, allylic and homoallylic alcohols 20,22,24,26,28,30,33 and α,β-unsaturated ketones 35,37,39. Generally, the epoxidation proceeds in a peracid-like manner with greater diastereoselectivity than those by common oxidants. In particular, the epoxidation of Δ4 3-ketosteroids 39a-i led to 4α,5α-epoxides
-
Synthesis of Symmetrical and Hybrid Dimeric Steroids by Double Suzuki–Miyaura Cross Coupling of 4-Bromo-3-oxo Steroids and Benzene-1,4-diboronic Acid作者:Martha C. Mayorquín-Torres、Gerardo I. Santiago-Sampedro、Marcos Flores-Álamo、Martín. A. Iglesias-ArteagaDOI:10.1055/s-0037-1611484日期:2019.8one hybrid dimer in which the steroid cores are connected by a 1,4-phenylene moiety were obtained by double Suzuki–Miyaura cross coupling of benzene-1,4-diboronic acid with 4-bromo-4-en-3-oxosteroids derived from cholesterol and diosgenin. Detailed NMR characterization of the obtained dimers is described. Two symmetrical dimers and one hybrid dimer in which the steroid cores are connected by a 1,4-phenylene
-
Synthesis of Highly Functionalized Allylic Alcohols from Vinyl Oxiranes and <i>N</i>-Tosylhydrazones via a Tsuji–Trost-Like “Palladium–Iodide” Catalyzed Coupling作者:Stefano Parisotto、Annamaria DeagostinoDOI:10.1021/acs.orglett.8b03026日期:2018.11.2An unprecedented efficient palladium(0)-catalyzed reaction of vinyl oxiranes with N-tosylhydrazones, affording “skipped” allylic alcohols in total regioselectivity and stereoselectivity, with excellent functional-group compatibility, is reported. In this Tsuji–Trost-like allylation, we propose the use of N-tosylhydrazones as a synthetic equivalent of α-styryl anions. More than 20 new products are described
-
�ber Steroide und Sexualhormone. 155. Mitteilung. �ber 3?, 5-Dioxy-koprostan und zwei epimere 3,4-Dioxy-cholestane作者:Pl. A. Plattner、H. Heusser、A. B. KulkarniDOI:10.1002/hlca.19480310647日期:——Durch katalytische Reduktion des Cholestenon-epoxyds ist es gelungen, auf partialsynthetischem Weg 3α, 5-Dioxy-koprostan zu bereiten, das in der räumlichen Anordnung des Asymmetriezentrums 5 der Konfiguration des Strophanthidins und verwandter Aglykone entspricht.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B