[4-(aminomethyl)cyclohexyl]methanamine | 10029-09-1
中文名称
——
中文别名
——
英文名称
[4-(aminomethyl)cyclohexyl]methanamine
英文别名
cis-cyclohexane-1,4-diyldimethanamine;cis-1,4-cyclohexane-bis(methylamine);cis-1,4-bis(aminomethyl)cyclohexane;C,C'-cyclohexane-1,4-diyl-bis-methylamine;cis-1,4-Bis-aminomethyl-cyclohexan;cis-1,4-cyclohexanedimethanamine;cis-1,4-cyclohexylenebis(methylamine)
CAS
10029-09-1
化学式
C8H18N2
mdl
——
分子量
142.244
InChiKey
OXIKYYJDTWKERT-OCAPTIKFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-9°C(lit.)
-
沸点:76°C/0.9mmHg(lit.)
-
密度:0.912±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.71
-
重原子数:10.0
-
可旋转键数:2.0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:52.04
-
氢给体数:2.0
-
氢受体数:2.0
安全信息
-
危险等级:8/6.1
-
海关编码:2921300090
-
储存条件:存放于惰性气体中,避免与空气接触。
SDS
cis-1,4-Bis(aminomethyl)cyclohexane Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: cis-1,4-Bis(aminomethyl)cyclohexane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 3
Acute toxicity (Dermal)
Skin corrosion/irritation Category 1B
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Harmful if swallowed
Toxic in contact with skin
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Do not breathe dust/fume/gas/mist/vapours/spray.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Remove/Take off immediately all contaminated clothing.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
cis-1,4-Bis(aminomethyl)cyclohexane
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: cis-1,4-Bis(aminomethyl)cyclohexane
Percent: >98.0%(GC)(T)
CAS Number: 10029-09-1
Synonyms: cis-1,4-Di(aminomethyl)cyclohexane , cis-Hexahydro-p-xylylenediamine
Chemical Formula: C8H18N2
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water in large amounts, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
cis-1,4-Bis(aminomethyl)cyclohexane
Section 7. HANDLING AND STORAGE
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Almost colorless
Odour: No data available
pH: No data available
Melting point/freezing point:-9°C
Boiling point/range: 76°C/0.1kPa
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.94
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents, Acids
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
cis-1,4-Bis(aminomethyl)cyclohexane
Section 12. ECOLOGICAL INFORMATION
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
8: Corrosive.
Hazards Class:
Subsidiary risk: 6.1: Toxic substance.
2922
UN-No:
Proper shipping name: Corrosive liquid, toxic, n.o.s.
II
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: cis-1,4-Bis(aminomethyl)cyclohexane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 3
Acute toxicity (Dermal)
Skin corrosion/irritation Category 1B
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Harmful if swallowed
Toxic in contact with skin
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Do not breathe dust/fume/gas/mist/vapours/spray.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Remove/Take off immediately all contaminated clothing.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
cis-1,4-Bis(aminomethyl)cyclohexane
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: cis-1,4-Bis(aminomethyl)cyclohexane
Percent: >98.0%(GC)(T)
CAS Number: 10029-09-1
Synonyms: cis-1,4-Di(aminomethyl)cyclohexane , cis-Hexahydro-p-xylylenediamine
Chemical Formula: C8H18N2
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water in large amounts, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
cis-1,4-Bis(aminomethyl)cyclohexane
Section 7. HANDLING AND STORAGE
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Almost colorless
Odour: No data available
pH: No data available
Melting point/freezing point:-9°C
Boiling point/range: 76°C/0.1kPa
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.94
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents, Acids
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
cis-1,4-Bis(aminomethyl)cyclohexane
Section 12. ECOLOGICAL INFORMATION
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
8: Corrosive.
Hazards Class:
Subsidiary risk: 6.1: Toxic substance.
2922
UN-No:
Proper shipping name: Corrosive liquid, toxic, n.o.s.
II
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,4-环己烷双(甲基胺) 1,4-bis(aminomethyl)cyclohexane 2549-93-1 C8H18N2 142.244 —— cis-cyclohexane-dicarboxylic acid-(1.4)-diamide 70925-16-5 C8H14N2O2 170.211 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— C-(4-methylene-cyclohexyl)-methylamine 854444-57-8 C8H15N 125.214
反应信息
-
作为反应物:描述:[4-(aminomethyl)cyclohexyl]methanamine 在 sodium hydride 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 38.0h, 生成 3,3'-cis-cyclohexane-1,4-diyldimethyl-bis-oxazolidin-2-one参考文献:名称:进一步评估N,N'-聚亚甲基桥连的2-氨基乙硫醇衍生物和相关化合物作为辐射防护剂。摘要:DOI:10.1021/jm00294a019
-
作为产物:描述:cis-cyclohexane-1,4-dicarbonitrile 在 氨 、 氢气 作用下, 以 甲醇 为溶剂, 50.0 ℃ 、1.2 MPa 条件下, 生成 [4-(aminomethyl)cyclohexyl]methanamine参考文献:名称:乙酰辅酶A羧化酶1和2的小分子抑制剂的设计显示了肥胖Zucker大鼠体内肝丙二酰辅酶A水平的降低摘要:抑制乙酰辅酶A羧化酶具有调节长链脂肪酸生物合成和线粒体脂肪酸氧化的潜力。ACC2弱抑制剂的杂交提供了一个新颖的,中等效力但亲脂的系列。优化产生化合物33和37,它们显示出对人ACC2的有效抑制作用,选择性比对人ACC1的抑制作用高10倍,具有良好的物理和体外ADME性质以及良好的生物利用度。X射线晶体学表明该系列结合在ACC2的CT域中,并揭示了两个关键的氢键相互作用。两个33倍37下部的水平在体内在肥胖Zucker大鼠肝丙二酰-CoA。DOI:10.1016/j.bmc.2011.04.014
文献信息
-
METHOD FOR PRODUCING TRANS-BIS(AMINOMETHYL)CYCLOHEXANE, METHOD FOR PRODUCING BIS(ISOCYANATOMETHYL)CYCLOHEXANE, BIS(ISOCYANATOMETHYL)CYCLOHEXANE, POLYISOCYANATE COMPOSITION, AND POLYURETHANE RESIN申请人:MITSUI CHEMICALS, INC.公开号:US20160207875A1公开(公告)日:2016-07-21A method for producing trans-bis(aminomethyl)cyclohexane includes a trans-isomerization step in which cis-dicyanocyclohexane is isomerized into trans-dicyanocyclohexane by heating dicyanocyclohexane containing cis-dicyanocyclohexane in the presence of a tar component produced by distillation of dicyanocyclohexane; and an aminomethylation step in which trans-dicyanocyclohexane produced by the trans-isomerization step is allowed to contact with hydrogen to produce trans-bis(aminomethyl)cyclohexane.
-
N,N'-Heptamethylenebis(4-methoxybenzamide)申请人:Sterling Drug Inc.公开号:US04009208A1公开(公告)日:1977-02-224-(Q-O)-4'-R.sub.1 -N,N'-alkylenebis(benzamides), N,N'-alkylenebis(3,4-methylenedioxybenzamides) or N,N'-alkylenebis[4-(lower-alkoxy)benzamides], having endocrinological properties, where Q is lower-alkyl, lower-alkoxyalkyl, lower-alkenyl, halo-lower-alkyl, halo-lower-alkenyl, lower-cycloalkyl, phenyl and BN-(lower-alkyl) where BN is di-(lower-alkyl)amino or a saturated N-heteromonocyclic radical having from five to seven ring atoms and alkylene has at least five carbon atoms between its two connecting linkages and R.sub.1 is Q-O-, hydrogen, lower-alkoxy, lower-alkyl, halo, benzyloxy, hydroxy, di-(lower-alkyl)amino, nitro, amino or trihalomethyl are prepared preferably by reacting the appropriate diamine or N-(aminoalkyl)-benzamide with two or one molar equivalents, respectively, of the appropriate benzoyl halide.
-
N,N'-bridged-bis[2-alkyl-2-hydroxyethylamines]申请人:Sterling Drug Inc.公开号:US04022833A1公开(公告)日:1977-05-10N,N'-Bridged-bis[(O and/or N-substituted)-2-alkyl-2-hydroxyethylamines] of the formula ##STR1## are prepared by condensing an epoxide of the formula ##STR2## and a diamine of the formula R'NH-X-NHR'. The products and dicarbanilates, acid-addition salts, N,N'-dioxides and N,N'-diammonium quaternary salts derived therefrom have antibacterial activity in vitro and are useful as antibacterial agents.
-
DINUCLEOTIDE COMPOUNDS FOR HCV INFECTION申请人:IDENIX PHARMACEUTICALS, INC.公开号:US20140112886A1公开(公告)日:2014-04-24Provided herein are compounds, compositions and methods for the treatment of Flaviviridae infections, including HCV infections. In certain embodiments, compounds and compositions of nucleoside derivatives are disclosed, which can be administered either alone or in combination with other anti-viral agents. In certain embodiments, the compounds comprise two 2′-methyl nucleotides linked according to Formula I: N 1 -L-N 2 (I) or a pharmaceutically acceptable salt, ester, solvate, stereoisomer, isomeric form, tautomeric form or polymorphic form thereof; wherein N 1 , L and N 2 are as described herein.本文提供了用于治疗黄病毒科感染,包括HCV感染的化合物、组合物和方法。在某些实施方式中,披露了核苷类衍生物的化合物和组合物,可以单独或与其他抗病毒药物联合给药。在某些实施方式中,所述化合物包括根据以下式I连接的两个2'-甲基核苷酸:N1-L-N2(I)或其药学上可接受的盐、酯、溶剂合物、立体异构体、同分异构体、互变异构体或其多形形式;其中N1、L和N2如本文所述。
-
METHOD FOR PRODUCING TRANS-1,4-BIS(AMINOMETHYL) CYCLOHEXANE申请人:Yoshimura Naritoshi公开号:US20130197270A1公开(公告)日:2013-08-01A method for producing trans-1,4-bis(aminomethyl)cyclohexane includes a nuclear hydrogenation step of producing a hydrogenated terephthalic acid or terephthalic acid derivative by nuclear hydrogenation of a terephthalic acid or terephthalic acid derivative, the terephthalic acid or terephthalic acid derivative being at least one selected from the group consisting of terephthalic acid, terephthalic acid ester, and terephthalic acid amide; a cyanation step of treating the hydrogenated terephthalic acid or terephthalic acid derivative with ammonia, thereby producing 1,4-dicyanocyclohexane, and producing trans-1,4-dicyanocyclohexane from the obtained 1,4-dicyanocyclohexane; and an aminomethylation step of treating the trans-1,4-dicyanocyclohexane with hydrogen, thereby producing trans-1,4-bis(aminomethyl)cyclohexane. Metal oxide is used as a catalyst in the cyanation step, and the obtained trans-1,4-dicyanocyclohexane has a metal content of 3000 ppm or less.
表征谱图
-
氢谱1HNMR
-
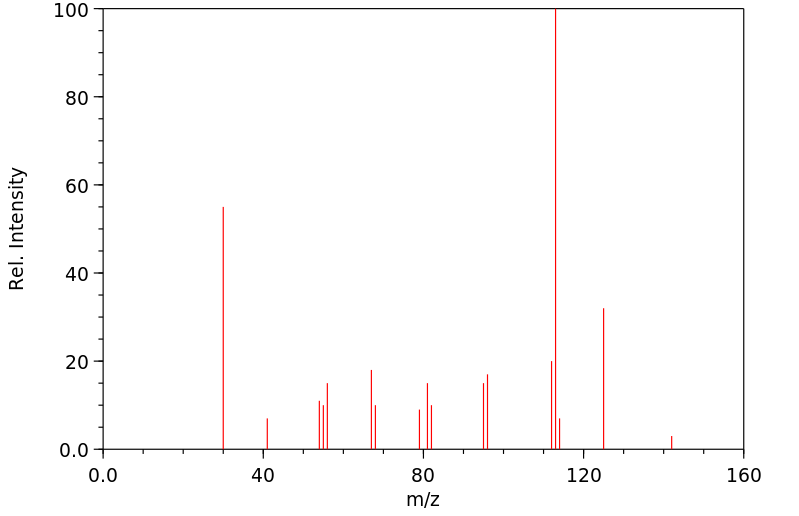
质谱MS
-
碳谱13CNMR
-
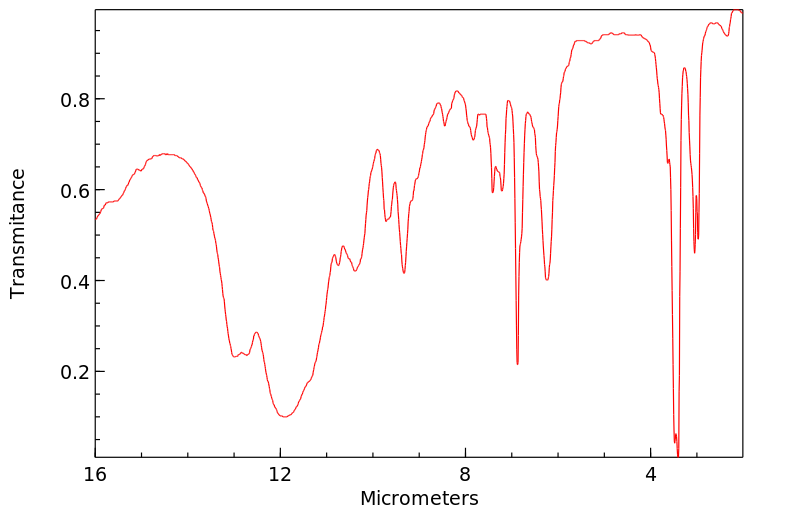
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷