(E)-1,2-dimethoxy-4-(2-nitroprop-1-enyl)benzene | 37629-53-1
中文名称
——
中文别名
——
英文名称
(E)-1,2-dimethoxy-4-(2-nitroprop-1-enyl)benzene
英文别名
(E)-1,2-dimethoxy-4-(2-nitroprop-1-en-1-yl)benzene;1,2-dimethoxy-4-((E)-2-nitroprop-1-enyl)benzene;(E)-1-(3,4-dimethoxyphenyl)-2-nitropropene;3.4-dimethoxy-1-(2-nitro-cis-propenyl)-benzene;3.4-Dimethoxy-1-(2-nitro-propen-(1)-yl-(1c))-benzol;3.4-Dimethoxy-1-(2-nitro-cis-propenyl)-benzol;3,4-Dimethoxy-beta-methyl-beta-nitrostyrene;1,2-dimethoxy-4-[(E)-2-nitroprop-1-enyl]benzene
CAS
37629-53-1
化学式
C11H13NO4
mdl
——
分子量
223.229
InChiKey
JGFBGRHDJMANRR-SOFGYWHQSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74 °C
-
沸点:349.3±27.0 °C(Predicted)
-
密度:1.168±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:16
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:64.3
-
氢给体数:0
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2-二甲氧基-4-丙-1-烯-2-基苯 4-isopropenyl-1,2-dimethoxybenzene 30405-75-5 C11H14O2 178.231
反应信息
-
作为反应物:描述:(E)-1,2-dimethoxy-4-(2-nitroprop-1-enyl)benzene 在 lithium aluminium tetrahydride 作用下, 以 甲醇 、 乙醚 、 苯 为溶剂, 生成 benzylidene-[2-(3,4-dimethoxy-phenyl)-1-methyl-ethyl]-amine参考文献:名称:3,4-二甲氧基苯丙胺及其N-烷基衍生物的一些N-氧化产物的合成摘要:描述了3,4-二甲氧基苯丙胺及其N-甲基和N-苄基衍生物的N-单烷基羟胺,N,N-二烷基羟胺,硝酮,恶唑烷,亚硝基和其他N-氧化产物的合成,包括新的简单方法制备N,N-二烷基羟胺以及分离和分析α-未取代的硝酮的方法。DOI:10.1016/0040-4020(75)80276-6
-
作为产物:参考文献:名称:Bruckner, Justus Liebigs Annalen der Chemie, 1935, vol. 518, p. 226,241摘要:DOI:
文献信息
-
Novel dihydroquinolizinones for the treatment and prophylaxis of hepatitis B virus infection申请人:Hoffmann-La Roche Inc.公开号:US20150210682A1公开(公告)日:2015-07-30The invention provides novel compounds having the general formula: wherein R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are as described herein, compositions including the compounds and methods of using the compounds.这项发明提供了具有一般公式的新化合物: 其中R1、R2、R3、R4、R5和R6如本文所述,包括这些化合物的组合物和使用这些化合物的方法。
-
[EN] PHENETHYLAMIDE DERIVATIVES AND THEIR HETEROCYCLIC ANALOGUES<br/>[FR] DÉRIVÉS DE PHÉNÉTHYLAMIDE ET LEURS ANALOGUES HÉTÉROCYCLIQUES申请人:ACTELION PHARMACEUTICALS LTD公开号:WO2010044054A1公开(公告)日:2010-04-22The invention relates to novel phenethylamide derivatives and their heterocyclic analogues of formula (I), wherein A, B, R1, R2 and R3 are as described in the application, and to the use of such compounds, or of pharmaceutically acceptable salts of such compounds, as medicaments, especially as orexin receptor antagonists.
-
Instantaneous SmI2/H2O/amine mediated reduction of nitroalkanes and α,β-unsaturated nitroalkenes作者:Tobias Ankner、Göran HilmerssonDOI:10.1016/j.tetlet.2007.05.105日期:2007.8A rapid method for efficient reduction of nitroalkanes and α,β-unsaturated nitroalkenes using SmI2/H2O/amine has been developed.已经开发出一种使用SmI 2 / H 2 O /胺有效还原硝基链烷和α,β-不饱和硝基链烯的快速方法。
-
Ethylenediamine: A Highly Effective Catalyst for One-Pot Synthesis of Aryl Nitroalkenes via Henry Reaction and Dehydration作者:Jianxin Yang、Jing Dong、Xia Lü、Qiang Zhang、Wei Ding、Xiaoxin ShiDOI:10.1002/cjoc.201201094日期:2012.12Ethylenediamine (H2NCH2CH2NH2) was found to be a highly effective catalyst for the condensation of aryl aldehydes with nitromethane (or nitroethane). When 1%–2% (mol%) of ethylenediamine was used as the catalyst, the one‐pot reaction of aryl aldehydes with nitromethane (or nitroethane) by refluxing for 3–10 h efficiently afforded various arylnitroalkenes 1a–1y in 85%–97% yields.
-
Enantioselective hydrogenation of α,β-disubstituted nitroalkenes作者:Shengkun Li、Kexuan Huang、Xumu ZhangDOI:10.1039/c4cc03942d日期:——The first highly chemo- and enantioselective hydrogenation of α,β-disubstituted nitroalkenes was accomplished with rhodium/JosiPhos-J2 as a catalyst, with the yield and enantioselectivity of up to 95% and 94%, respectively. The α-chiral nitroalkanes will provide an entry to valuable chiral amphetamines which are otherwise not so easily accessed.
表征谱图
-
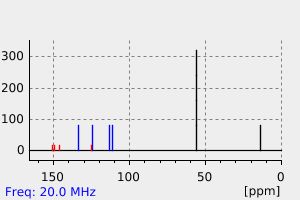
氢谱1HNMR
-
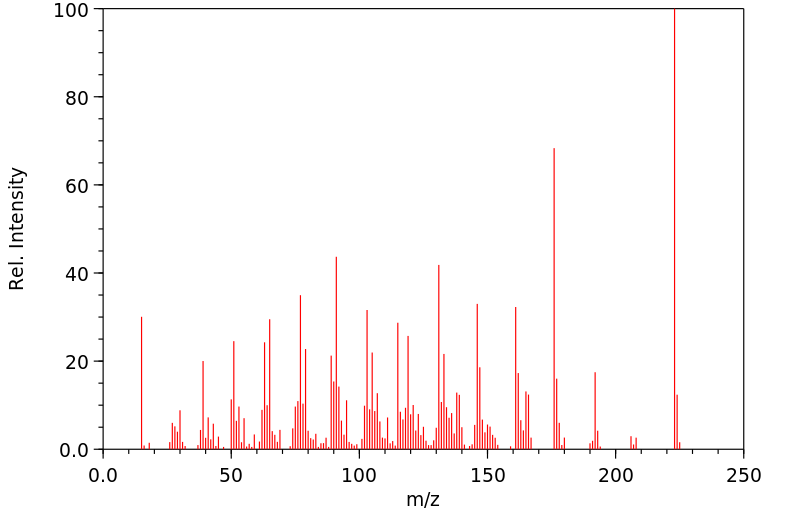
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫