Cu(tropolonate)2 | 15663-10-2
中文名称
——
中文别名
——
英文名称
Cu(tropolonate)2
英文别名
Cu(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium)2;Cu(trp)2;bis(tropolonato)copper(II);Bis(tropolonato)copper;copper;7-oxocyclohepta-1,3,5-trien-1-olate
CAS
15663-10-2
化学式
C14H10CuO4
mdl
——
分子量
305.777
InChiKey
JBUPAQOHIYVYBH-UHFFFAOYSA-L
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:187-189 °C
计算性质
-
辛醇/水分配系数(LogP):0.24
-
重原子数:19
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:80.3
-
氢给体数:0
-
氢受体数:4
SDS
反应信息
-
作为反应物:描述:参考文献:名称:醇盐桥接铜 (II) Hinokitiolato 和 Tropolonato 配合物:多态性、重建相变和磁性摘要:通过单晶和粉末 X 射线衍射研究了 [Cu(trop)(μ-OMe)]2 (trop = tropolonate) 中的多晶型和意外重构相变;相变与 ca 的巨大滞后有关。200℃。在易于重现的晶体形式中,甲醇桥连的双核亚基通过 Jahn-Teller 扭曲的配位球中的更长键聚集成无限链。具有取代配体桧醇 (hino)、[Cu(hino)(μ-OR)]2 (R = Me、Et、iPr) 的类似醇盐桥接衍生物形成双核复合物对并聚集成离散的四核分子。阳离子间距离模式反映在这两种结构类型的磁性中:在两种情况下都观察到双核亚基内的强反铁磁耦合,DOI:10.1002/zaac.201500742
-
作为产物:描述:参考文献:名称:铜(II)-马酚酮复合物对人乳腺癌细胞,乳房多细胞球体和乳腺球的抗癌和抗转移活性。摘要:这项工作的目的是展示铜(II)与曲托酮(trp),复合物[Cu(trp)2]对单层(2D)和球形(3D)中人乳腺癌细胞的抗癌和抗转移活性。针对MCF7(IC50(复合物)= 5.2±1.8μM,IC50(CDDP)= 19.3±2.1μM)和MDA-MB-231(IC50(复合物)= 4.0±0.2μM,IC50(CDDP)= 27.0±1.9)的细胞毒性测定μM)证明[Cu(trp)2]在2D和3D人乳腺癌细胞模型上比顺铂(CDDP)发挥更大的抗肿瘤作用。此外,[Cu(trp)2]通过降低金属蛋白酶活性抑制细胞迁移,并且该化合物以较低的浓度(2.5-10μM)经历乳腺癌细胞的凋亡。此外,[Cu(trp)2]克服了CDDP,相对于乳房多细胞球体,IC50值降低了26倍((IC50(复合物)= 4.9μM,IC50(CDDP)= 130μM))。此外,我们的结果表明[Cu(trp)2]抑制了DOI:10.1016/j.jinorgbio.2019.110975
文献信息
-
Induced Circular Dichroism of Bis(tropolonato)copper(II) with Optically Active Amines作者:Tasuku MurakamiDOI:10.1246/bcsj.58.3411日期:1985.11Bis(tropolonato)copper(II) forms adducts with chiral aromatic amines and exhibits circular dichroism(CD) induced for d-d and intra-ligand transitions. The applicability of the dispersion-induced CD theory to this complex system is discussed.Bis(tropolonato)copper(II) 与手性芳香胺形成加合物,并表现出对 dd 和配体内跃迁诱导的圆二色性 (CD)。讨论了色散诱导 CD 理论对这个复杂系统的适用性。
-
Synthesis structure magnetic properties of [Cu5(bta)6L4] (bta=benzotriazolate;L=β-diketonate) Clusters作者:Mark Murrie、David Collison、C.David Garner、Madeleine Helliwell、Peter A Tasker、Scott S TurnerDOI:10.1016/s0277-5387(98)00161-2日期:1998.8Reaction of [CuL2] (LH: tfpbdH = 4,4,4-trifluoro-1-phenyl-1,3-butanedione; tropH = tropolone; or acacH =acetylacetone; Ph/Me-pdH = I-phenyl-l,3-butanedionel Ph/Ph-pdH = 1,3-diphenyl-1,3-propanedione; or C4H3S/CF3-pdH =4,4,4-trifluoro-1-(2-thienyl-1,3-butanedione)) with benzotriazole (btaH) in CH2Cl2 yields the corresponding compound [Cu-5(bta)(6)L-4] (L = tfpbd, 1; trop, 2; acac, 3; (Ph/Me-pd) 4 (Ph/Ph-pd) 5; (C4H3S/CF3-pd) 6). The structures of 1 and 2 have been determined by single crystal X-ray diffraction revealing a distorted tetrahedral arrangement of four Cu(II) centered on a fifth Cu(II). Each of the six bta ligands spans an edge of the Cu, tetrahedron and is ligated to the central Cu through its central nitrogen. Solid-state magnetic susceptibility studies from 2-300 K have been accomplished for 1, 2 and 3 and reveal a weak antiferromagnetic intramolecular interaction between the five Cu(II) centres. The tridentate (mu(3)-bridging) bonding motif of bta is well suited for the formation of a protective monolayer of bta(-) ions on oxidised copper surfaces. Furthermore, the flexibility in this mode of coordination exhibited by bta(-) ions, as manifest within the structures of 1, 2, and 3 (the structure of which was determined previously), is advantageous and would allow benzotriazolate to adapt to the various surface topographies which could well be presented by lightly oxidized copper surfaces. (C) 1998 Elsevier Science Ltd. All rights reserved.
-
FEMI-ONADEKO BANKOLE; KOMOLAFE OLUSEGUN OLUWOLE, EGYPT. J. PHARM. SCI., 26,(1985) N 1-4, 191-194作者:FEMI-ONADEKO BANKOLE、 KOMOLAFE OLUSEGUN OLUWOLEDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
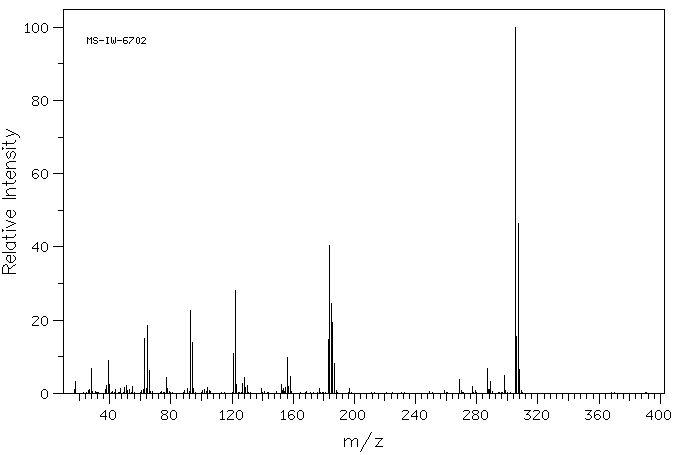
质谱MS
-
碳谱13CNMR
-
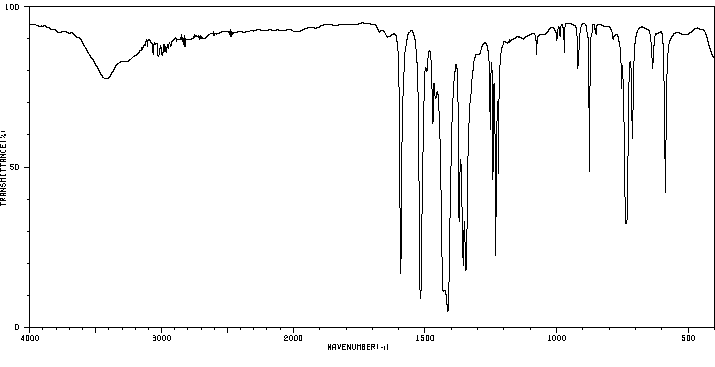
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-