3-bromo-2-methoxytropone | 3054-77-1
中文名称
——
中文别名
——
英文名称
3-bromo-2-methoxytropone
英文别名
3-Brom-2-methoxytropon;3-bromo-2-methoxycyclohepta-2,4,6-trien-1-one
CAS
3054-77-1
化学式
C8H7BrO2
mdl
——
分子量
215.046
InChiKey
NEJKTXSCJWBCJQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:76 °C
-
沸点:297.8±40.0 °C(Predicted)
-
密度:1.54±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-溴-2-羟基-2,4,6-环庚三烯-1-酮 3-bromo-2-hydroxy-2,4,6-cycloheptatrien-1-one 4584-68-3 C7H5BrO2 201.019 2-甲氧基-2,4,6-环庚三烯-1-酮 2-methoxytropone 2161-40-2 C8H8O2 136.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,7-dibromo-2-methoxy-cycloheptatrienone 33695-99-7 C8H6Br2O2 293.942
反应信息
-
作为反应物:参考文献:名称:Seto, Sci. Rep. Tohoku Univ., Ser. 1: Phys., Chem., Astron., 1953, vol. <1> 37, p. 275,282摘要:DOI:
-
作为产物:描述:环庚三烯酚酮 在 N-溴代丁二酰亚胺(NBS) 、 18-冠醚-6 、 potassium carbonate 作用下, 以 四氯化碳 为溶剂, 反应 4.0h, 生成 3-bromo-2-methoxytropone参考文献:名称:WO2019200314A5摘要:公开号:WO2019200314A5
文献信息
-
The Grignard Reaction of 3-Halotropolone Methyl Ethers作者:Haruki Tsuruta、Toshio MukaiDOI:10.1246/bcsj.41.2489日期:1968.10The Grignard reaction of the methyl ethers (I and III) of 3-bromotropolone and those (II and IV) of 3-chlorotropolone with methylmagnesium iodide or phenylmagnesium bromide were investigated. When I or II was allowed to react with methylmagnesium iodide, 2-methoxyphenyldimethylcarbinol (V) and 2-methoxy-6-isopropylphenol (VI) were obtained. The reaction of I or II with phenylmagnesium bromide afforded
-
Cyclohepta[<i>b</i>][1,4]benzoxazines 3. The Reaction of 6-Bromocyclohepta[<i>b</i>][1,4]benzoxazine with<i>o</i>-Aminophenol作者:Tetsuo Nozoe、Harue Okai、Hidetsugu Wakabayashi、Sumio IshikawaDOI:10.1246/bcsj.62.2307日期:1989.7The reaction of 6-bromocyclohepta[b][1,4]benzoxazine (19) and o-aminophenol (3) was studied and compared with that of 3-bromo-2-methoxytropone (2) with 3. 14H-[1,4]Benzoxazino[3′,2′:3,4]cyclohepta[1,2-b][1,4]benzoxazine, 10-(o-hydroxyanilino)cyclohepta[b][1,4]benzoxazine (D), 1- and 4-formylphenoxazines and their Schiff bases (C and X) were obtained in very good yields by modifying the conditions of the reaction of 19 with 3. Structures of D, C, and their isomers were determined and the possible reaction pathways for the formation of various products are discussed.
-
The Bromination of 2-Methoxytropone and Its Bromo Derivatives作者:Tetsuo Nozoe、Kahei Takase、Masafumi YasunamiDOI:10.1246/bcsj.44.2218日期:1971.8various solvents. The treatment of 2-methoxytropone or 5-bromo-2-methoxytropone with bromine in methanol gave an addition product, 2,6-dibromo-7,7-dimethoxy- (II) or 2,4,6-tribromo-7,7-dimethoxy-2,4-cycloheptadien-1-one (XIV) respectively, together with the substitution products. On the contrary, the treatment of 2-methoxytropone with bromine in anhydrous ethanol, chloroform, or carbon tetrachloride gave
-
Reactive Troponoids and<i>o</i>-Aminophenol. V. The Reaction of 3-Bromo-2-methoxytropone and<i>o</i>-Aminophenol作者:Taichi Someya、Harue Okai、Hidetsugu Wakabayashi、Tetsuo NozoeDOI:10.1246/bcsj.56.2756日期:1983.9The reaction products of the title substances in hot acetic acid were separated by preparative TLC into compounds A–L according to the Rf-values, and the structural assignments for these products were now made as follows: A, 14H-[1,4]benzoxazino[3′,2′ : 3,4]cyclohepta[1,2-b][1,4]benzoxazine; B, 1-formylphenoxazine; F, cyclohepta[2,1-b : 2,3-b′]di[1,4]benzoxazine; G, cyclohepta[b][1,4]benzoxazin-10(11H)-one or its enolic form; J, cyclohepta[b][1,4]benzoxazin-6(11H)-one; L, 6-(o-hydroxyanilino)cyclohepta[b][1,4]benzoxazine hydrobromide. A small amount of 2-methylamino-3H-phenoxazin-3-one was also produced. Possible reaction pathways for the formation of these products are also discussed.标题物质在热醋酸中的反应产物经制备型 TLC 根据 Rf 值分成 A-L 化合物,这些产物的结构分配如下:A, 14H-[1,4]benzoxazino[3′,2′ : 3,4]cyclohepta[1,2-b][1,4]benzoxazine; B, 1-formylphenoxazine; F, cyclohepta[2,1-b :G,环庚烷并[b][1,4]苯并恶嗪-10(11H)-酮或其烯醇形式;J,环庚烷并[b][1,4]苯并恶嗪-6(11H)-酮;L,6-(邻羟基苯胺基)环庚烷并[b][1,4]苯并恶嗪氢溴酸盐。此外,还生成了少量 2-甲基氨基-3H-苯并恶嗪-3-酮。此外,还讨论了形成这些产物的可能反应途径。
-
Nozoe et al., Proceedings of the Japan Academy, 1951, vol. 27, p. 18,21作者:Nozoe et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
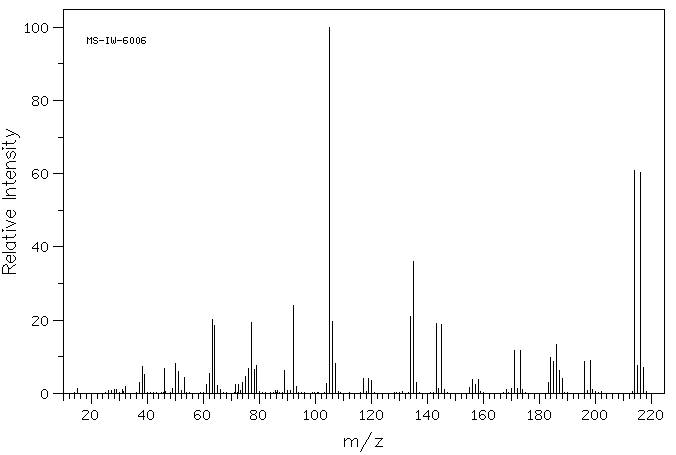
质谱MS
-
碳谱13CNMR
-
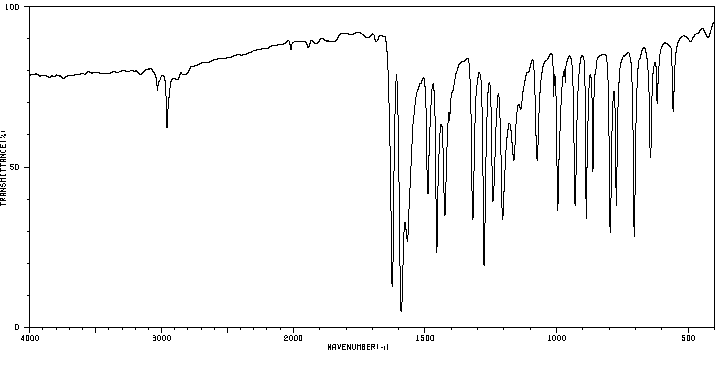
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-