3,4-bis(diphenylmethylene)-1,2-cyclobutanedione | 23596-82-9
中文名称
——
中文别名
——
英文名称
3,4-bis(diphenylmethylene)-1,2-cyclobutanedione
英文别名
3,4-bis(diphenylmethylene)-cyclobutanedione;3,4-bis(diphenylmethylene)cyclobutanedione;3.4-Bis-(diphenylmethylen)-cyclobutandion-(1.2);3,4-Bis-(diphenylmethylen)-cyclobutan-1,2-dion;3,4-Bis-(diphenylmethylen)-cyclobuta-1,2-dion;3.4-Bis-diphenylmethylen-cyclobutandion-(1.2);3,4-Bis(diphenylmethylidene)cyclobutane-1,2-dione;3,4-dibenzhydrylidenecyclobutane-1,2-dione
CAS
23596-82-9
化学式
C30H20O2
mdl
——
分子量
412.488
InChiKey
PLQSOQJQSXUCLN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:625.5±65.0 °C(Predicted)
-
密度:1.240±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):7.3
-
重原子数:32
-
可旋转键数:4
-
环数:5.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
SDS
反应信息
-
作为反应物:描述:3,4-bis(diphenylmethylene)-1,2-cyclobutanedione 在 氨 、 溴 作用下, 以 四氯化碳 为溶剂, 以34%的产率得到3,4-bis(diphenylmethylene)succinimide参考文献:名称:Structure, solid-state photochemistry and reactivity in asymmetric synthesis of 3,4-bis(diphenylmethylene)-N-methylsuccinimide摘要:The synthesis and structural features of 3,4-dihydro-3,4-bis(diphenylmethylene)-N-methylsuccinimide [3,4-bis(diphenylmethylene)-1-methyl-2,5-pyrrolidinedione (6b), C31H23NO1, M(r)=441.53] are described. (6b) crystallizes at room temperature in three polymorphic forms: (A) monoclinic P2(1), a=11.640(3), b=9.257(2), c=12.103(4)Angstrom, beta=114.83(1)degrees, V=1183.6(6)A(3) Z=2, F(000)=464, D-x=1.239gcm(-3), mu=0.72cm(-1), R(F)=0.077 for 1560 observations [I>3 sigma(I)]; (B) orthorhombic, Pbcn, a=9.964(1), b=20.181(3), c=11.622(3)Angstrom, V=2337.0(7)Angstrom(3), Z=4, F(000)=928, D-x=1.255gcm(-3), mu=0.73cm(-1), R(F)=0.044 for 1466 observations [I>3 sigma(I)]; (C) monoclinic, P2(1)/n, a=9.485(3), b=11.014(2), c=22.945(3)Angstrom, V=2369.9(9)Angstrom(3), Z=4, F(000)=928, D-x=1.238gcm(-3), mu=0.72cm(-1), R(F)=0.060 for 2200 observations [I>3 sigma(I)]. The (6b) molecule adopts a helical (prochiral) configuration with approximate C-2 symmetry in order to accommodate the steric hindrance between the aryl substituents; the conformation is very similar in the three polymorphs. Transformations between the different polymorphs can be induced easily, and it is possible to vary the amount of chiral polymorph in the crystallization mixture by seeding techniques and the use of acidic additives. Crystalline (6b) undergoes a photocyclization reaction to yield N-methyl-1,1,4-triphenyl-1,2-dihydronaphthalene-2,3-dicarboximide (8b). In the observed conformation of (6b), the intramolecular distances between the unsaturated carbon sites which join during the photochemical reaction are within 3.27-3.38 Angstrom. The solid-state photoreaction of the chiral polymorph A gives an optically active molecular product (8b) of 64% enantiomeric excess (e.e.), and represents a chiral enrichment process assisted by the crystal medium. The corresponding photochemical reactions with the racemic polymorphs B and C yield racemic (8b).DOI:10.1107/s0108768194014540
-
作为产物:参考文献:名称:环丁二烯醌的亚甲基类似物-II:2-卤代和2,2-二卤代-3,4-双(二苯基亚甲基)环丁酮的制备和反应摘要:已经制备了标题化合物(II,XVII;二氯和二溴衍生物),并且描述了化合物的一些新反应。DOI:10.1016/s0040-4020(01)98071-8
文献信息
-
THE SYNTHESIS OF 3-BENZOYL-4-METHYLENECYCL0BUT-1-ENE AND 6-METHYLENE-1-OXASPIRO[2,3]HEX-4-ENE, AND THEIR RING EXPANSION INTO METHYLENECYCLOPENTENONES作者:Kozaburo Ueda、Fumio TodaDOI:10.1246/cl.1974.359日期:1974.4.5The reaction of 2,3,3-tribromo-1-diphenylhydroxymethyl-4-diphenylmethylenecyclobut-1-ene (I) with AgClO4 afforded new 4,5-dibromo-2,2-diphenyl-6-diphenylmethylene-1-oxaspiro [2,3]hex-4-ene (VI) and 3-benzoyl-1,2-dibromo-3-phenyl-4-diphenylmethylenecyclobut-1-ene (VIII), probably via the methylenecyclobutenylium ion (II). New acid-catalyzed ring expansion reaction of VI to afford 3,4-dibromo-2,2-diphenyl-5-diphenylmethylenecyclopent-3-en-1-one (XIV) and 2,3-dibromo-5,5-diphenyl-4-diphenylmethylenecyclopent-2-en-1-one (XV) was found.
-
The Oxidation of 1,3-Butadienes with Nitric Acid into 2,5-Dihydrofurans作者:Fumio Toda、Eishiro TodoDOI:10.1246/bcsj.47.348日期:1974.2The oxidation of 3,4-bis(diphenylmethylene)-succinic anhydride (I), -1-benzylsuccinimide (VI), and -γ-butyrolactone (VIII) with nitric acid afforded the corresponding 2,5-dihydrofuran derivatives, III, VII, and IX respectively. The same oxidation of 3,4-bis(diphenylmethylene)cyclobutane-1,2-dione (XI) afforded 3,4-bis(diphenylhydroxymethyl)cyclobut-3-ene-1,2-dione (XIII), probably via the bicyclic cyclobutenedione intermediate (XII).
-
Methylene Analogs of Cyclobutenedione. VI. Interconversion between 3,4-Dimethylcyclobut-3-ene-1,2-dione and 3,4-Dimethylenecyclobutane-1,2-dione作者:Fumio Toda、Nobuhiro OoiDOI:10.1246/bcsj.46.1733日期:1973.6enolate anion (VIII), afforded III and IV in various ratios depending on the steric nature of the substituent R. Bromination of IV in 99% acetic acid afforded 3,4-bis(hydroxydiphenylmethyl)cyclobut-3-ene-1,2-dione (XI). Although the bromination of XI with phosphorus tribromide afforded IV, that with bromine afforded the 2,5-dihydrofuran derivative (XVII). The reaction of I with o-phenylenediamine to afford在 ROH(R=H、Me、Et 和 i-Pr)中用溴处理 3,4-双(二苯基甲基)环丁-3-烯-1,2-二酮 (I),得到 3,4-双(二苯基亚甲基) )环丁烷-1,2-二酮 (IV) 和 3-二苯基甲基-4-烷氧基二-苯基甲基环丁-3-烯-1,2-二酮 (III) 的比例取决于 ROH 的 R 的空间体积。III 在 KOH-MeOH 中的溶液的酸化,其中 III 以烯醇阴离子 (VIII) 的形式存在,根据取代基 R 的空间性质以不同的比例提供 III 和 IV。 IV 在 99% 乙酸中的溴化得到3,4-双(羟基二苯基甲基)环丁-3-烯-1,2-二酮(XI)。虽然用三溴化磷对 XI 进行溴化得到 IV,但用溴化得到 2,5-二氢呋喃衍生物 (XVII)。
-
Condensations of 3,4-Bis(diphenylmethylene)-1,2-cyclobutanedione with<i>o</i>-Phenylenediamine under Ionic and Radical Reaction Conditions作者:Zaiko Ro、Koichi Tanaka、Fumio TodaDOI:10.1246/bcsj.56.3193日期:1983.10The title condensations under ionic and radical reaction conditions gave 1,2-bis(diphenylmethylene)-1,2-dihydrocyclobuta[b]quinoxaline and 7,8-bis(diphenylmethylene)-7,8-dihydrobenzo[b][1,4]diazocine-6,9(5H,10H)-dione respectively.
-
Isolation of tetraphenyl-, pentaphenyl-, hexaphenyl-, and heptaphenyl-substituted tetramethylenecyclobutanes. Stable tetraradialenes作者:Koichi Tanaka、Fumio TodaDOI:10.1016/s0040-4039(00)78587-x日期:1980.1
表征谱图
-
氢谱1HNMR
-
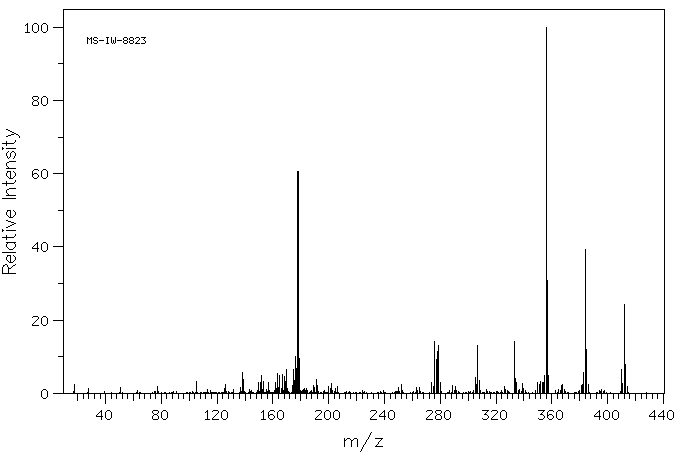
质谱MS
-
碳谱13CNMR
-
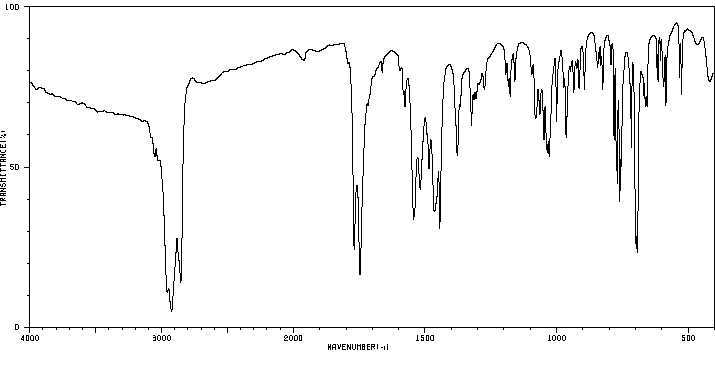
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫