5-氯-1-戊烯 | 928-50-7
中文名称
5-氯-1-戊烯
中文别名
2-溴乙基乙酸酯
英文名称
5-chloropent-1-ene
英文别名
5-chloro-1-pentene;5-chloropentene;4-pentenyl chloride
CAS
928-50-7
化学式
C5H9Cl
mdl
——
分子量
104.579
InChiKey
UPOBJNRMUDPATE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:104.85°C
-
密度:0.9072
-
保留指数:730
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:6
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903299090
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P210
-
危险品运输编号:1993
-
危险性描述:H225
-
储存条件:2-8°C,干燥密封保存。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-5-chloro-1-iodo-1-pentene 78461-58-2 C5H8ClI 230.476
反应信息
-
作为反应物:参考文献:名称:酰基自由基:σ自由基族中电子自旋共振谱,结构和稳定性之间的关系摘要:在流体溶液中已经产生了一系列饱和和不饱和的酰基R 1 O,主要是通过在相应的醛存在下对二叔丁基过氧化物进行光解。大部分的酰基的显示良好分辨的超精细分裂,和的值一(H β)关联与NMR耦合常数3 Ĵ(C ħ Ç ħ O)在父醛。结论是,酰基和相应的醛具有相似的结构。DOI:10.1039/p29800000819
-
作为产物:参考文献:名称:酰基自由基:σ自由基族中电子自旋共振谱,结构和稳定性之间的关系摘要:在流体溶液中已经产生了一系列饱和和不饱和的酰基R 1 O,主要是通过在相应的醛存在下对二叔丁基过氧化物进行光解。大部分的酰基的显示良好分辨的超精细分裂,和的值一(H β)关联与NMR耦合常数3 Ĵ(C ħ Ç ħ O)在父醛。结论是,酰基和相应的醛具有相似的结构。DOI:10.1039/p29800000819
文献信息
-
Efficient Palladium(0) supported on reduced graphene oxide for selective oxidation of olefins using graphene oxide as a ‘solid weak acid’作者:Xi Gao、Jianhao Zhou、Xinhua PengDOI:10.1016/j.catcom.2019.01.020日期:2019.3Selective oxidation of olefin derivatives to ketones has made innovative development over palladium(0) supported on reduced graphene oxide. Compared to traditional Wacker oxidation, the novel method offers an economical and environment-friendly option by using graphene oxide (GO) as a ‘solid weak acid’ instead of classical homogeneous catalysts like H2SO4 and CF3COOH. X-ray diffraction, X-ray photoelectron
-
A new synthesis of functionally, substituted hydroquinones
-
Ni-Catalyzed Carboxylation of Aziridines en Route to β-Amino Acids作者:Jacob Davies、Daniel Janssen-Müller、Dmitry P. Zimin、Craig S. Day、Tomoyuki Yanagi、Jonas Elfert、Ruben MartinDOI:10.1021/jacs.1c01916日期:2021.4.7pressure is disclosed. The protocol is characterized by its mild conditions, experimental ease, and exquisite chemo- and regioselectivity pattern, thus unlocking a new catalytic blueprint to access β-amino acids, important building blocks with considerable potential as peptidomimetics.
-
Synthesis of Isomerically Pure (<i>Z</i>)-Alkenes from Terminal Alkynes and Terminal Alkenes: Silver-Catalyzed Hydroalkylation of Alkynes作者:Mitchell T. Lee、Madison B. Goodstein、Gojko LalicDOI:10.1021/jacs.9b09336日期:2019.10.30molecules and often are used as intermediates in organic synthesis. Many alkenes exist in two stereoisomeric forms (E and Z), which have different structures and different properties. The selective formation of the two isomers is an important synthetic goal that has long inspired the development of new synthetic methods. However, the efficient synthesis of diastereopure, thermodynamically less stable, Z-alkenes
-
Free-Radical Carbo-Alkenylation of Olefins: Scope, Limitations and Mechanistic Insights作者:Redouane Beniazza、Virginie Liautard、Clément Poittevin、Benjamin Ovadia、Shireen Mohammed、Frédéric Robert、Yannick LandaisDOI:10.1002/chem.201605043日期:2017.2.16The three‐component free‐radical carbo‐alkenylation of electron‐rich olefins has been studied, varying the substitution pattern in the alkene, in the radical precursor and in the final acceptor. New vinylsulfones were also prepared and their reactivity investigated. The scope and limitations of the process was established, and the reaction mechanism clarified using selected dienes as radical clocks已经研究了富电子烯烃的三组分自由基碳烯基化反应,改变了烯烃,自由基前体和最终受体中的取代方式。还制备了新的乙烯基砜,并研究了它们的反应性。确定了该方法的范围和局限性,并使用选定的二烯作为自由基钟阐明了反应机理。因此,人们认识到,已释放的磺酰基可逆地加到烯烃上是一个重要事件,在使用这种多组分碳烯基化反应时不应忽视。
表征谱图
-
氢谱1HNMR
-
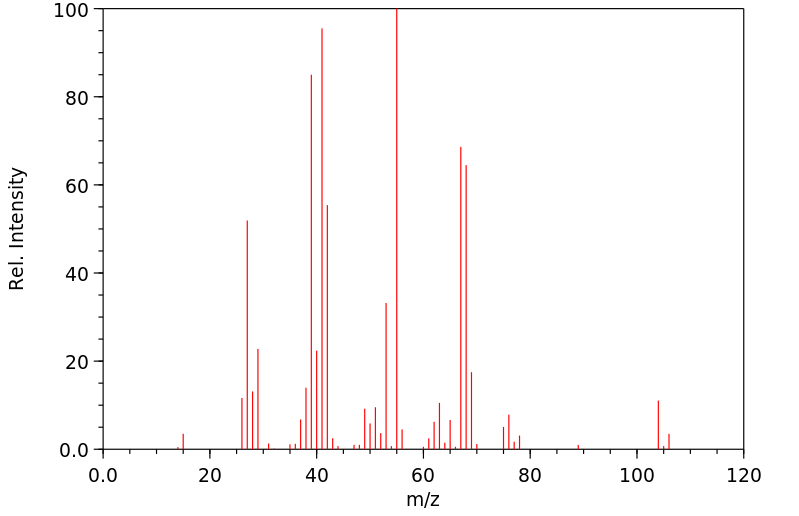
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷