6-甲基苯并(a)吩噻嗪-5-酮 | 26197-31-9
中文名称
6-甲基苯并(a)吩噻嗪-5-酮
中文别名
——
英文名称
6-Methyl-5H-benzo[a]phenothiazin-5-one
CAS
26197-31-9
化学式
C17H11NOS
mdl
——
分子量
277.346
InChiKey
RTPGWOYHPVTPOX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:20
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:54.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯并[a]吩噻嗪-5-酮 5H-Benzophenothiazin-5-one 25947-04-0 C16H9NOS 263.32
反应信息
-
作为反应物:描述:6-甲基苯并(a)吩噻嗪-5-酮 生成 5-hydroxy-6-methyl-12H-benzo[a]phenothiazine参考文献:名称:GUINDON, YVAN;GIRARD, YVES;LAU, CHEUK K.;FORTIN, REJEAN;ROKACH, JOSHUA;YO+摘要:DOI:
-
作为产物:参考文献:名称:Benzo[α]phenoxazines and benzo[α]phenothiazine from vitamin K3: synthesis, molecular structures, DFT studies and cytotoxic activity摘要:从维生素K3中提取的新型苯并[α]苯醌噻唑和苯并[α]苯并噻唑对HeLa、MCF-7细胞系具有细胞毒性,并有潜在的拓扑异构酶II抑制剂作用。DOI:10.1039/c5ra08496b
文献信息
-
Phenothiazone derivatives and analogs申请人:Merck Frosst Canada, Inc.公开号:US04667032A1公开(公告)日:1987-05-19Phenothiazone derivatives and analogs thereof, pharmaceutical compositions and methods of treatment are disclosed. These compounds are useful as inhibitors of mammalian leukotriene biosynthesis. As such, these compounds are useful therapeutic agents for treating allergic conditions, asthma, cardiovascular disorders and inflammation.
-
Benzo[A]phenothiazines and hydro-derivatives申请人:Merck Frosst Canada, Inc.公开号:US04611056A1公开(公告)日:1986-09-09Pharmaceutical compositions containing a compound of Formula I: ##STR1## wherein X is O, S, SO or SO.sub.2 and R.sub.2, R.sub.3, R.sub.4 and R.sub.5 may be positioned anywhere on the structure, or a pharmaceutically-acceptable salt thereof and certain novel benzo[a]phenothiazines, which compositions and compounds are useful in treating allergic conditions, asthma, cardiovascular disorders, inflammation and pain and are useful as cytoprotective agents.含有Formula I化合物的药物组合物:其中X为O、S、SO或SO.sub.2,R.sub.2、R.sub.3、R.sub.4和R.sub.5可以位于结构的任何位置,或其药用盐,以及某些新型苯并[a]苯并噻嗪类化合物,这些组合物和化合物可用于治疗过敏症、哮喘、心血管疾病、炎症和疼痛,并可用作细胞保护剂。
-
Benzo[a]phenothiazines and hydro-derivatives for inhibiting leukotriene申请人:Merck Frosst Canada, Inc.公开号:US04876246A1公开(公告)日:1989-10-24Pharmaceutical compositions containing a compound of Formula I: ##STR1## wherein X is O, S, SO or SO.sub.2 and R.sub.2, R.sub.3, R.sub.4 and R.sub.5 may be positioned anywhere on the structure, or a pharmaceutically-acceptable salt thereof and certain novel benzo[a]phenothiazines, which compositions and compounds are useful in treating allergic conditions, asthma, cardiovascular disorders, inflammation and pain and are useful as cytoprotective agents.含有公式I中化合物的药物组合物:其中X为O,S,SO或SO.sub.2,而R.sub.2,R.sub.3,R.sub.4和R.sub.5可以位于结构的任何位置,或其药学上可接受的盐和某些新型苯并[a]苯并噻吩,这些组合物和化合物用于治疗过敏性疾病,哮喘,心血管疾病,炎症和疼痛,并且作为细胞保护剂有用。
-
Benzo[a]phenothiazines and hydro-derivatives and pharmaceutical compositions containing them申请人:MERCK FROSST CANADA INC.公开号:EP0136893A2公开(公告)日:1985-04-10Pharmaceutical compositions contain a compound for the Formula or a pharmaceutically acceptable salt thereof. Certain compounds of Formula I are novel. Compounds of Formula I are useful in treating allergic conditions, asthma, cardrovascular disorders, inflammation and pain and are useful as cytoprotective agents. In Formula 1. X is O, S, SO or SO2; R, is H; C,-6 alkyl; C, -6 acyl; C1-6 aminoacyl; (C1-6 acyloxy)-(C1-6 alkyl); (C1-6 alkoxy)-(Cl-6 alkyl); benzoyl; substituted benzoyl in which the substitution in the phenyl ring (herein called "substituted as herein defined") is halogen, C1-3 alkyl, C1-3 alkoxy, CN, CF3, COOR6, OH, CH2COOR6 or (CH2)nNR8R9 where n is 0, 1 or 2; carbamoyl; CONHR7; COOR7; p-toluenesulfonyl; methane sulfonyl; or an acyl group such that R,-OH is an essential amino acid; each of R2, R3, R4 and R5, independently of the others, is hydrogen; C1-6 alkyl; C2-6 alkenyl; -(CHR6)pCOOR6, p being 0 or an integer from 1 to 4; or -(CH2)mM where m is 0 or an integer from 1 to 6 and M is (a) OR,s; (b) halogen; (c) CF3; (d) SR15; (e) phenyl or substituted phenyl as herein defined; (f) COOR6; (g) CO-R14; (h) tetrazolyl; (i) -NH-CO-R7; (j) -NR8R9; (k) -NHSO2R10 where R10 is OH, C1-6 alkyl, C1-6 alkoxy, or phenyl; (I) -COCH20H; (m) -SOR11 where R11 is C1-6 alkyl; phenyl; substituted phenyl as herein defined; (CH2)mCOOR6;CN; formyl or C1-4 perfluoroalkyl; (n) -CONR8R9; (o) -SO2NR8R9; (p) -SO2R13 where R13 is a hydrogen atom or a radical of the type defined for R11; (q) NO2; (r) O-CO-R14; (s) O-CO-NR8R9; (t) -CN; or (u) -OPO(OR6)2: each R6, independently of any other, is H, phenyl or C1-6 alkyl; each R7, independently of any other, is C1-6 alkyl, benzyl, phenyl or (C1-6 acyloxyl-(C1-6 alkyl); each R8 and each R9, independently of any other, is phenyl, substituted phenyl as herein defined, or C1-4 alkyl, or NR8R9 represents a heterocycloalkyl radical of 5 to 8 ring atoms; each R14, independently of any other, is H. (CH2)pCOOR6, C, 6 alkyl, C1-6 alkoxy, (C1-6 acyloxy)-(C1-6 alkoxy), phenyl, substituted phenyl as herein defined, or C, -6 aminoalkyl, or R14 is such that R14CO2H is an essential amino acid; R15 is H, (C1-6 alkoxy)-(C1-6 alkyl), (C, 6 acyloxyl-(C1-6 alkyl), C1-6 alkyl, benzyl, -(CH2)mCOOR6, CN, formyl, C1-4 perfluoroalkyl, CH2-R12 where R12 is C1-5 alkyldimethylamino or phenyl, phenyl, or substituted phenyl as herein defined; the broken lines in ring A represent optional double bonds; R2, R3, R4 and R5 may be positioned anywhere in the structure.药用组合物包含一种式化合物 或其药学上可接受的盐。式 I 的某些化合物是新型化合物。式 I 的化合物可用于治疗过敏性疾病、哮喘、心血管疾病、炎症和疼痛,并可用作细胞保护剂。 在式 1 中 X 是 O、S、SO 或 SO2; R,是 H;C,-6 烷基;C, -6酰基;C1-6氨基酰基;(C1-6酰氧基)-(C1-6烷基);(C1-6烷氧基)-(Cl-6烷基);苯甲酰基;取代的苯甲酰基,其中苯环中的取代基(此处称为 "如本文定义的取代基")是卤素、C1-3烷基、C1-3烷氧基、CN、CF3、COOR6、OH、 COOR6 或 (CH2)nNR8R9 其中 n 是 0、1 或 2;氨基甲酰基;CONHR7;COOR7;对甲苯磺酸基;甲烷磺酰基;或 R,-OH 为必需氨基酸的酰基; R2、R3、R4 和 R5 中各自独立地为氢;C1-6 烷基;C2-6 烯基;-(CHR6)pCOOR6,p 为 0 或 1 至 4 的整数;或-( )mM,其中 m 为 0 或 1 至 6 的整数,M 为 (a) OR,s;(b) 卤素; (c) ; (d) SR15; (e) 本文定义的苯基或取代苯基; (f) COOR6; (g) CO-R14; (h) 四唑基; (i) -NH-CO-R7; (j) -NR8R9;(k) -NHSO2R10 其中 R10 为 OH、C1-6 烷基、C1-6 烷氧基或苯基; (I) -CO 0H; (m) -SOR11 其中 R11 为 C1-6 烷基;苯基;本文定义的取代苯基;( )mCOOR6; CN; 甲酰基或 C1-4 全氟烷基;(n) -CONR8R9; (o) -SO2NR8R9 ; (p) -SO2R13 其中 R13 是氢原子或 R11 所定义的基团类型; (q) NO2; (r) O-CO-R14; (s) O-CO-NR8R9; (t) -CN;或 (u) -OPO(OR6)2: 每个 R6(独立于任何其他物质)是 H、苯基或 C1-6 烷基; 每个 R7(独立于任何其他物质)是 C1-6 烷基、苄基、苯基或 (C1-6 acyloxyl-(C1-6 烷基); 每个 R8 和每个 R9,各自独立地为苯基、本文定义的取代苯基或 C1-4 烷基,或 NR8R9 代表 5 至 8 个环原子的杂环烷基; ( )pCOOR6 是 H、( )pCOOR6、C, 6 烷基、C1-6 烷氧基、(C1-6 乙酰氧基)-(C1-6 烷氧基)、苯基、本文定义的取代苯基或 C, -6 氨基烷基,或 R14 是这样的 R14CO2H 是必需氨基酸; R15 是 H、(C1-6 烷氧基)-(C1-6 烷基)、(C, 6 乙酰氧基-(C1-6 烷基))、C1-6 烷基、苄基、-( )mCOOR6、CN、甲酰基、C1-4 全氟烷基、 -R12,其中 R12 是本文定义的 C1-5 烷基二甲基氨基或苯基、苯基或取代苯基; 环 A 中的断线代表可选的双键; R2、R3、R4 和 R5 可位于结构中的任何位置。
-
Use of lipoxygenase inhibitors for the preparations of cytoprotective pharmaceutical compostions and process for the preparation of a cytoprotective pharmaceutical composition申请人:Merck & Co., Inc.公开号:EP0155623A2公开(公告)日:1985-09-25Lipoxygenase inhibitors are used as cytoprotective active ingredient in producing cytoprotective pharmaceutical compositions. Corresponding cytoprotective pharmaceutical compositions are prepared by incorporating into a pharmaceutical composition a lipoxygenase inhibitor in a cytoprotective active amount. The prepared cytoprotective therapeutic composition may be used to treat or prevent disease states such as erosive gastritis, erosive esophagitis, inflammatory bowel disease, and induced hemorrhagic gastric erosions such as those caused by indomethacin or ethanol. The lipoxygenase inhibitors induce cytoprotection via an unspecified mechanism.
表征谱图
-
氢谱1HNMR
-
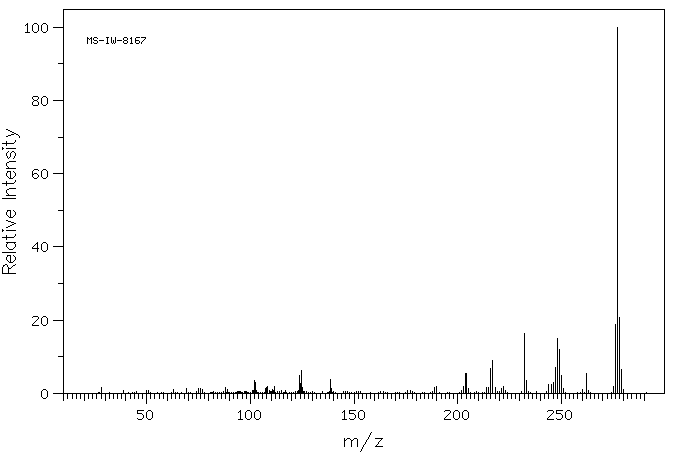
质谱MS
-
碳谱13CNMR
-
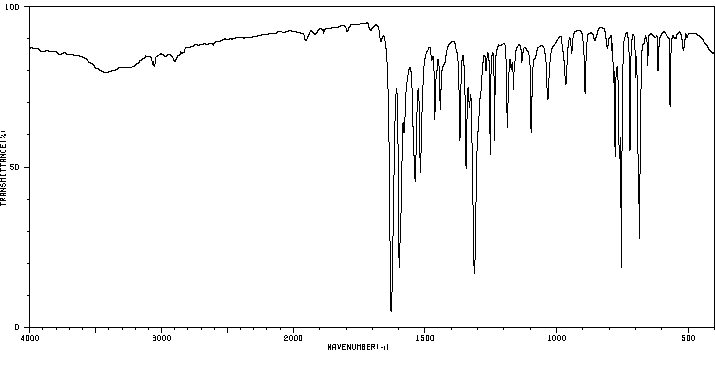
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高氟奋乃静
马来酸甲哌丙嗪
马来酸奋乃静
马来酸乙巯拉嗪
锁匹达新
醋酸奋乃静
醋异丙嗪
酒石酸异丁嗪
还原亚甲蓝
达赛马嗪
螺氯丙嗪
莫雷西嗪亚砜
茶氯酸异丙嗪
苹果酸硫乙拉嗪
苯达莫司汀杂质A
苯甲酸2-(2H-1,4-苯并噻嗪-3-基)酰肼
苯甲酸,4-硝基-2-[[3-(三氟甲基)苯基]氨基]-
苯甲酰基氧基甲基-[3-(2-氯吩噻嗪-10-基)丙基]-二甲基氯化铵
苯并噻嗪-5-氧化
苯并噻嗪-5-正离子,3,7-二(二甲氨基)-4-碘-,氯化
苯并噻嗪,10-(2-(4-丙基-1-哌嗪基)丙基)-
苯并[b]吩噻嗪-12-基(苯基)甲酮
苯并[a]吩噻嗪-5-酮
苯丙嗪
苄酰基无色亚甲基兰
芬诺宁
芬乙嗪
舒多昔康
羟乙哌氟嗪
美索哒嗪
美索丙嗪
美洛昔康钾盐
美洛昔康钠
美洛昔康-d3
美洛昔康
美托奋乃酯
美托哌丙嗪酸
美托哌丙嗪
美托咪嗪-d6
美喹他嗪亚砜
美喹他嗪
磺达嗪
硫堇(劳氏紫)
硫利达嗪杂质A(EP)
硫利达嗪N-氧化物
硫利达嗪-5-亚砜
硫利达嗪
硫代哒嗪-d35-亚砜
硫丙拉嗪
盐酸诺美丙嗪