2-acetyl-4-methylbenzo[b]thiophene | 1467-88-5
中文名称
——
中文别名
——
英文名称
2-acetyl-4-methylbenzo[b]thiophene
英文别名
2-Acetyl-4-methylbenzo(b)thiophene;1-(4-methyl-1-benzothiophen-2-yl)ethanone
CAS
1467-88-5
化学式
C11H10OS
mdl
——
分子量
190.266
InChiKey
JWDNVFPOFKNDEE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为产物:描述:2-Methylmercapto-6-methyl-benzaldehyd 、 一氯丙酮 在 magnesium oxide 作用下, 以 甲苯 为溶剂, 反应 4.0h, 以95.7%的产率得到2-acetyl-4-methylbenzo[b]thiophene参考文献:名称:Investigation on the One-Step Preparation of 2-Substituted Benzo[B]Thiophenes摘要:A convenient and efficient synthesis of 2-substituted benzo[b]thiophenes is described. This one-step reaction of alkylthiobenzaldehyde and activated electrophiles proceeded smoothly in toluene at 120 degrees C with magnesium oxide as a catalyst. The target 2-substituted benzo[b]thiophenes are obtained in moderate yields and the side reaction is inhibited via azeotropic distillation. According to the experiments, a plausible mechanism is presented.DOI:10.1080/10426507.2012.717140
文献信息
-
Efficient Method for Synthesis of 2-Acetylbenzo(b)thiophene and Its Derivatives, the Key Synthons for 5-Lipoxygenase Inhibitors作者:Sanjay R. Chemburkar、David G. Anderson、Rajarathnam E. ReddyDOI:10.1080/00397910903162775日期:2010.6.16tert-butylmercaptan (6) to form 2-(tert-butylthio)benzaldehyde (7a), which upon treatment with HBr in water gave the disulfide derivative 2,2′-disulfanediyldibenzaldehyde (8a) in 97% yield. Finally, the reaction of 8a with acetylacetone (9) and 1-chloroacetone (10) gave 2-acetylbenzo(b)thiophene (2a) in 94% yield. The methodology is general and suitable for the preparation of its derivatives, 2b–d.
表征谱图
-
氢谱1HNMR
-
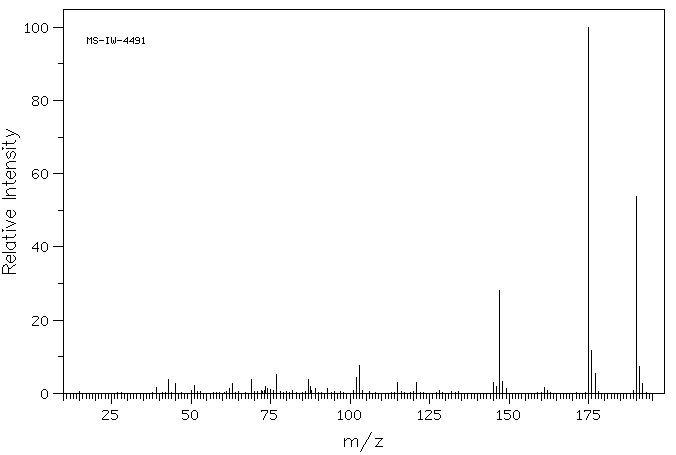
质谱MS
-
碳谱13CNMR
-
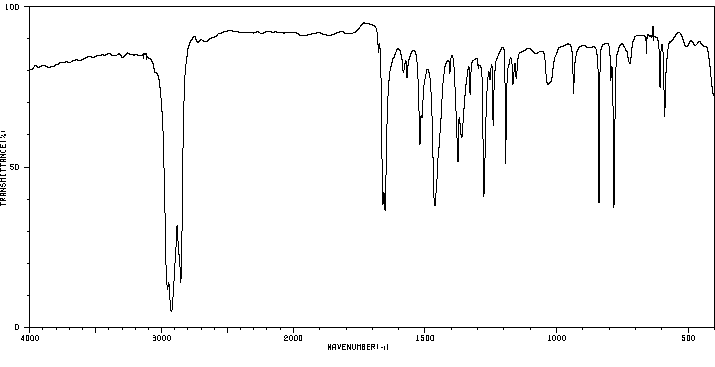
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯