MDM乙内酰脲 | 16228-00-5
中文名称
MDM乙内酰脲
中文别名
单甲羟基二甲基乙酰内脲;单甲羟基二甲基乙酰内脲结晶;3-羟甲基-5,5-二甲基乙内酰脲;乙内酰脲
英文名称
3-hydroxymethyl-5,5-dimethylhydantoin
英文别名
2,4-Imidazolidinedione, 3-(hydroxymethyl)-5,5-dimethyl-;3-(hydroxymethyl)-5,5-dimethylimidazolidine-2,4-dione
CAS
16228-00-5
化学式
C6H10N2O3
mdl
——
分子量
158.157
InChiKey
IVQCQIORBPXQGP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:1.257±0.06 g/cm3(Predicted)
-
物理描述:Liquid
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:69.6
-
氢给体数:2
-
氢受体数:3
反应信息
-
作为反应物:参考文献:名称:Surface active N-Halamine compounds摘要:提供了N-卤胺生物杀菌材料和涂层。单体的噁唑烷酮或海达因酮与其他单体共聚或均聚,以产生在暴露于氯或溴溶液后具有生物杀菌作用的材料或涂层。这些生物杀菌材料和涂层在表面接触时有效地灭活微生物,并在进一步暴露于氯或溴溶液后失去效力时可再生。可以用这些材料和涂层处理的表面包括但不限于:玻璃、塑料、金属、纤维和木材,用于游泳池和储罐衬里、食品包装、导管、油漆、瓷砖、淋浴墙、织物、无菌绷带、管道、医疗和牙科涂层、防腐剂等。公开号:US06469177B1
文献信息
-
[EN] SUBSTITUTED QUINOLINES AND THEIR USE AS MEDICAMENTS<br/>[FR] QUINOLÉINES SUBSTITUÉES ET LEUR UTILISATION COMME MÉDICAMENTS申请人:BOEHRINGER INGELHEIM INT公开号:WO2013014060A1公开(公告)日:2013-01-31The invention relates to new substituted quinolines of formula (1) wherein R1 is a linear or branched C1-6-alkyl, wherein R1 may optionally be substituted by R3 which is selected from the group consisting of a three-, four-, five-, six- or seven-membered cycloalkl; a five-, six- or seven-membered, saturated heterocycle comprising one, two or three heteroatoms each independently selected from the group consisting of N, S and O; and a five- or six-membered heteroaryl comprising one, two or three heteroatoms each independently selected from the group consisting of N, S and O; wherein R3 may optionally be substituted further substituted as defined in claim 1 and wherein R2 is selected from the group consisting of halogen, phenyl, a five- or six-membered monocyclic heteroaryl comprising one, two or three heteroatoms each independently selected from the group consisting of N, S and O; a bicyclic, nine-, ten- or eleven-membered, either aromatic or non-aromatic, but not fully saturated heterocycle comprising one, two, three or four heteroatoms each independently selected from the group consisting of N, S and O; wherein R2 may optionally be further substituted as defined in claim 1, and their use in the preparation of medicaments for the treatment of disease such as asthma, COPD, allergic rhinitis, allergic dermatitis and rheumatoid arthritis.该发明涉及公式(1)中的新取代喹啉,其中R1是直链或支链的C1-6-烷基,其中R1可以选择性地被R3取代,R3选自以下组:三、四、五、六或七元环烷基;含有一个、两个或三个异原子(分别独立地选自N、S和O)的五、六或七元饱和杂环;含有一个、两个或三个异原子(分别独立地选自N、S和O)的五元或六元杂芳基;其中R3可以选择性地进一步取代,如权利要求书中所定义,R2选自以下组:卤素、苯基、含有一个、两个或三个异原子(分别独立地选自N、S和O)的五元或六元单环杂芳基;含有一个、两个、三或四个异原子(分别独立地选自N、S和O)的九、十或十一元双环芳香或非芳香但不完全饱和杂环;其中R2可以选择性地进一步取代,如权利要求书中所定义,并且它们在制备用于治疗哮喘、慢性阻塞性肺病、过敏性鼻炎、过敏性皮炎和类风湿性关节炎等疾病的药物中的用途。
-
Surface active N-halamine compounds申请人:Auburn University公开号:US20030064051A1公开(公告)日:2003-04-03N-halamine biocidal materials and coatings are provided. Monomeric oxazolidinones or hydantoins are homopolymerized or copolymerized with other monomers so as to produce materials or coatings, which upon exposure to solutions of chlorine or bromine become biocidal. The biocidal materials and coatings are effective at inactivating microorganisms upon surface contact and are regenerable following loss of efficacy upon further exposure to solutions of chlorine or bromine. Surfaces which could be treated with the materials and coatings include, but are not limited to: glass, plastic, metals, fibers, and wood for use in pool and tank liners, food wrappers, catheters, paints, tiles, shower walls, fabrics, sterile bandages, pipes, medical and dental coatings, preservatives, and the like.
-
A composition and process for treating hair申请人:SYNTEX (U.S.A.) INC.公开号:EP0091740A2公开(公告)日:1983-10-19The composition comprises a water soluble bisulfite salt, a cationic surfactant penetrating agent, unsubstituted or substituted imidazolidinedione, and a water soluble organic acid salt buffered to a pH between 5.0-6.5. The process for treating hair comprises applying said composition to the hair, shaping the hair and, after an appropriate time, rinsing the hair with water.
-
Process for preparing methylolated hydantoins申请人:LONZA INC.公开号:EP0391645A1公开(公告)日:1990-10-10A method for making methylolated hydantoins by reacting a hydantoin, a formaldehyde such as paraformaldehyde and an alkaline catalyst at conditions of temperature and pressure sufficient to induce reaction to obtain a product in essentially 100% active form. Dry methylolated hydantoin product made by this process may be stored or shipped more economically than an aqueous solution containing a methylolated hydantoin.
-
Process for preparing methylolhydantoins申请人:LONZA INC.公开号:EP0571904A1公开(公告)日:1993-12-01There are provided methods for producing solid anhydrous methylolhydantoins. Initially, an aqueous stirrable medium is dehydrated and heated to yield a substantially anhydrous stirrable melt. The aqueous stirrable medium includes (A) a solute selected from the group consisting of (i) a dimethyloldimethylhydantoin, (ii) a hydantoin reactant, (iii) a formaldehyde source reactant, or (iv) a combination of any of the foregoing; and (B) optionally a catalyst. A molten system is then provided by adding, to the stirrable melt, a reactant mixture which includes (A)(i) the same or a different hydantoin reactant, (ii) a substantially anhydrous formaldehyde source reactant which may be the same as or different than the formaldehyde source reactant, or (iii) a combination thereof; and (B) optionally, the same or a different catalyst; wherein the molten system includes (i) at least one hydantoin reactant and (ii) at least one dehydrated formaldehyde source reactant or substantially anhydrous formaldehyde source reactant. The at least one hydantoin reactant and the at least one dehydrated formaldehyde source reactant or substantially anhydrous formaldehyde source reactant in the molten system are reacted, while reaction water is removed, to yield anhydrous molten methylolhydantoin. Finally, the molten methylolhydantoin is solidified. Alternatively, solid anhydrous methylolhydantoin is produced by reacting, in an aqueous stirrable medium as above, a reactant mixture of (i) the same or a different hydantoin reactant, (ii) a substantially anhydrous formaldehyde source reactant which may be the same as or different than the formaldehyde source reactant, or (ii) a combination thereof and (iv) optionally, the same as or different catalyst; wherein (i) at least one hydantoin reactant and (ii) at least one formaldehyde source reactant or substantially anhydrous formaldehyde reactant is present; while heating to at least the melting temperature of the methylolhydantoin and while removing substantially all water; to yield a molten methylolhydantoin; and subsequently solidifying the molten methylolhydantoin. These methods can be practiced as batch processes or as semi-continuous processes.本发明提供了生产固体无水甲基海因的方法。首先,对含水可搅拌介质进行脱水和加热,以得到基本无水的可搅拌熔体。水性可搅拌介质包括:(A) 选自以下组别的溶质:(i) 二甲基二羟甲基海因,(ii) 海因反应物,(iii) 甲醛源反应物,或 (iv) 上述任一物质的组合;以及 (B) 可选催化剂。然后通过向可搅拌熔体中加入反应物混合物来提供熔融体系,该反应物混合物包括 (A)(i)相同或不同的海因反应物,(ii)与甲醛源反应物相同或不同的基本无水甲醛源反应物,或 (iii) 上述反应物的组合;(B) 可选地,相同或不同的催化剂;其中熔融体系包括 (i) 至少一种海因反应物和 (ii) 至少一种脱水甲醛源反应物或基本无水甲醛源反应物。熔融体系中的至少一种海因反应物和至少一种脱水甲醛源反应物或基本无水甲醛源反应物发生反应,同时除去反应水,生成无水熔融甲基海因。最后,将熔融的甲基海因固化。另一种方法是,在上述水性可搅拌介质中,通过以下反应物混合物反应制得固态无水甲基海因:(i) 相同或不同的海因反应物;(ii) 与甲醛源反应物相同或不同的基本无水甲醛源反应物;或 (ii) 它们的组合;(iv) 可选的相同或不同的催化剂;其中(i)至少存在一种海因反应物和(ii)至少存在一种甲醛源反应物或基本无水的甲醛反应物;同时加热至至少甲醇海因的熔化温度,并除去基本所有的水;得到熔融的甲醇海因;随后将熔融的甲醇海因凝固。这些方法可以分批进行,也可以半连续进行。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
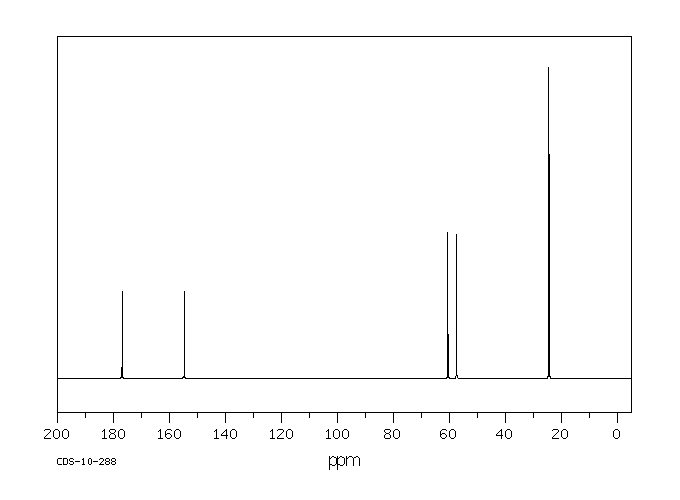
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)