N,N,N',N'-四乙基-1,3-丙二胺 | 60558-96-5
中文名称
N,N,N',N'-四乙基-1,3-丙二胺
中文别名
N,N,N',N'-四乙基-1,3-丙烷二胺;N,N,N’,N’-四乙基-1,3-丙二胺;N,N,N",N"-四乙基-1,3-丙二胺
英文名称
N,N',N,N'-tetraethyl-1,3-propanediamine
英文别名
N,N,N',N'-Tetraethyl-1,3-propanediamine;N,N,N',N'-tetraethylpropane-1,3-diamine
CAS
60558-96-5
化学式
C11H26N2
mdl
MFCD00009057
分子量
186.341
InChiKey
RCZLVPFECJNLMZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:157.5-159.5 °C
-
沸点:133 °C80 mm Hg(lit.)
-
密度:0.81 g/mL at 25 °C(lit.)
-
闪点:169 °F
-
稳定性/保质期:
避免接触氧化物
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:6.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S27,S36,S37,S39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2921290000
-
包装等级:III
-
危险品运输编号:UN 2735 8
-
危险标志:GHS05
-
危险性描述:H314
-
危险性防范说明:P280,P305 + P351 + P338,P310
-
储存条件:在密封的贮藏器中,应放置于阴凉、干燥处,并保持良好通风。
SDS
| Name: | N N N N -Tetraethyl-1 3-propanediamine 97% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 60558-96-5 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 60558-96-5 | N,N,N',N'-Tetraethyl-1,3-propanediamin | 97 | unlisted |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause cyanosis of the extremities. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May cause systemic effects.
Inhalation:
Causes chemical burns to the respiratory tract. The toxicological properties of this substance have not been fully investigated.
Aspiration may lead to pulmonary edema. May cause systemic effects.
Inhalation at high concentrations may cause CNS depression and asphixiation.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Combustible liquid.
Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use only in a well-ventilated area.
Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation.
Discard contaminated shoes. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 60558-96-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless - pale-yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 133 deg C @ 80 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 76 deg C ( 168.80 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 0.8100 g/ml
Molecular Formula: C11H26N2
Molecular Weight: 186.34
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 60558-96-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N,N,N',N'-Tetraethyl-1,3-propanediamine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: AMINES, LIQUID, CORROSIVE, N.O.S.*
Hazard Class: 8
UN Number: 2735
Packing Group: III
IMO
Shipping Name: AMINES, LIQUID, CORROSIVE, N.O.S.
Hazard Class: 8
UN Number: 2735
Packing Group: III
RID/ADR
Shipping Name: AMINES, LIQUID, CORROSIVE, N.O.S.
Hazard Class: 8
UN Number: 2735
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 25 Avoid contact with eyes.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 60558-96-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 60558-96-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 60558-96-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:N,N,N',N'-四乙基-1,3-丙二胺 以 二氯甲烷 为溶剂, 生成 [(N,N,N',N'-tetraethylpropane-1,3-diamine)2(μ-η2:η2-peroxodicopper(II))](CH3SO3)2参考文献:名称:Reversible O−O Bond Cleavage in Copper−Dioxygen Isomers: Impact of Anion Basicity摘要:Low-temperature oxygenation of copper(I) complexes of N,N,N',N'-tetraethylpropane-1,3-diamine yields solutions containing both mu-eta2:eta2-peroxodicopper(II) (P) and bis(mu-oxo)dicopper(III) (O) valence isomers. The P/O equilibrium position depends on the nature of the counteranion; P is favored with more basic anions. Titration and EXAFS experiments as well as DFT calculations suggest that axial donation from a sulfonate anion to the copper centers imparts an electronic/electrostatic bias toward the P isomer.DOI:10.1021/ja061132g
-
作为产物:描述:参考文献:名称:Reactions of Acrolein and Related Compounds. VI. Condensations with Amines摘要:DOI:10.1021/ja01128a044
文献信息
-
ANTIMICROBIAL COMPOSITIONS, METHODS OF PREPARATION THEREOF, AND USES THEREOF申请人:Coady Daniel J.公开号:US20120251607A1公开(公告)日:2012-10-04A composition of matter comprises a cationic polymer comprising a polycarbonate chain fragment, the polycarbonate chain fragment comprising a repeat unit comprising a side chain moiety containing a quaternary amine group; and a non-charged polymer comprising a polyester chain segment and a poly(alkylene oxide) chain segment; wherein i) the cationic polymer and the non-charged polymer are amphiphilic and biocompatible, ii) the cationic polymer and the non-charged polymer form a mixed complex by non-covalent interactions in water, and iii) the mixed complex is a more effective antimicrobial agent against at least a Gram-negative microbe compared to the cationic polymer and the non-charged polymer alone when tested using otherwise identical conditions.
-
[EN] COMPOSITIONS AND USES THEREOF<br/>[FR] COMPOSITIONS ET LEURS UTILISATIONS申请人:UNIV VIRGINIA PATENT FOUNDATION公开号:WO2019005883A1公开(公告)日:2019-01-03The disclosure is directed to compounds of the formula (IA), (I)-(V) and others disclosed herein and uses of such compounds.该披露涉及到公式(IA)、(I)-(V)和其他在此披露的化合物,以及这些化合物的用途。
-
[EN] PHOSPHORUS-CONTAINING COMPOUNDS USEFUL FOR MAKING HALOGEN-FREE, IGNITION-RESISTANT POLYMERS<br/>[FR] COMPOSÉS PHOSPHORÉS UTILES POUR LA FABRICATION DE POLYMÈRES RÉSISTANT À L'INFLAMMATION ET EXEMPTS D'HALOGÈNE申请人:DOW GLOBAL TECHNOLOGIES INC公开号:WO2005118604A1公开(公告)日:2005-12-15Phosphorus-containing compounds are disclosed which are obtainable by reacting: (A) at least one organophosphorus compound having a group selected from the group H-P= 0 ; the group P-H and the group P-OH; and (B) at least one compound having the following Formula (I): Formula (I) [R'(Y)m']m(X - O - R')n wherein R' is an organic group; Y is a functional group selected from hydroxy, carboxylic acid, carboxylate, acid 15 anhydride, and amine; -SH, -SO3H, -CONH2, -NHCOOR, phosphite and phosphinate groups X is a hydrocarbylene group; R' is hydrogen or a hydrocarbyl group having from 1 to 8 carbon atoms, R is alkyl or aryl group having from 1 to 12 carbon atoms; m', m and n are, independently, numbers equal to or greater than 1. These compounds are useful for making flame retardant epoxy and polyurethane resins and ignition resistant thermoplastic resins, each of which is useful for a variety of end uses requiring flame retardancy or ignition resistance. Flame retardant epoxy resins may be used to make electrical laminates. Flame retardant polyurethane is useful for making rigid polyurethane foam used in construction and flexible polyurethane foam used to make vehicle upholstery. Ignition resistant thermoplastic resins are useful for making television cabinets, computer monitors, printer housings, automotive parts, and housings and parts for appliances. This invention is particularly useful in end use applications in which a low bromine or low halogen content is required or desired.本发明揭示了一种含磷化合物,其可通过反应获得:(A)至少一种有机磷化合物,该有机磷化合物具有从H-P=0组中选择的基团;P-H基团和P-OH基团;和(B)至少一种具有以下化学式(I)的化合物:化学式(I) [R'(Y)m']m(X - O - R')n,其中R'是有机基团;Y是从羟基、羧酸、羧酸盐、酸酐和胺中选择的功能基团;-SH、-SO3H、-CONH2、-NHCOOR、磷酸酯和亚磷酸盐基团;X是一个烃基烷基;R'是氢或具有1至8个碳原子的烃基基团,R是具有1至12个碳原子的烷基或芳基基团;m'、m和n分别是大于或等于1的数字。这些化合物可用于制备阻燃环氧树脂和聚氨酯树脂以及防火热塑性树脂,每种树脂都可用于各种需要阻燃性或防火性的最终用途。阻燃环氧树脂可用于制造电气层压板。阻燃聚氨酯树脂可用于制造建筑中使用的刚性聚氨酯泡沫和用于制造汽车座椅的柔性聚氨酯泡沫。防火热塑性树脂可用于制造电视柜、计算机显示器、打印机外壳、汽车零部件以及电器外壳和零部件。本发明特别适用于需要或期望低溴或低卤素含量的最终用途应用中。
-
URIDINE MONOPHOSPHATE AND TRIPHOSPHATE DERIVATIVES, COMPOSITIONS AND METHODS OF USE申请人:Tufts University公开号:US20180085386A1公开(公告)日:2018-03-29This disclosure relates to uridine nucleoside derivatives, compositions comprising therapeutically effective amounts of those nucleoside derivatives and methods of using those nucleoside derivatives or compositions in treating disorders that are responsive to compounds, such as agonists, of P 2 Y 6 receptor, e.g., neuronal disorders, including neurodegenerative disorders (e.g., Alzheimer's disease, Parkinson's disease) and traumatic CNS injury, pain, Down Syndrome (DS), glaucoma and inflammatory conditions.
-
Pentaborate(1-) Salts and a Tetraborate(2-) Salt Derived from C2- or C3-Linked Bis(alkylammonium) Dications: Synthesis, Characterization, and Structural (XRD) Studies作者:Michael A. Beckett、Bashdar I. Meena、Thomas A. Rixon、Simon J. Coles、Peter N. HortonDOI:10.3390/molecules25010053日期:——The synthesis of a number of pentaborate(1-) salts from cations arising from N-substituted α,α-, α,β-, and α,γ-diaminoalkanes has been attempted in aqueous solution from B(OH)3 and the appropriate diammine in a 10:1 ratio. Despite relatively mild work-up conditions the pentaborate(1-) salts prepared were not always as anticipated and the following compounds were isolated in good yield: [Me2NH(CH2)2NHMe2][B5O6(OH)4]2已经尝试在水溶液中从 B(OH)3 和适当的 N-取代 α,α-、α,β- 和 α,γ-二氨基烷烃产生的阳离子合成许多五硼酸盐 (1-) 盐。以 10:1 的比例二胺。尽管处理条件相对温和,但制备的五硼酸盐 (1-) 盐并不总是如预期的那样,并且以良好的产率分离了以下化合物:[Me2NH(CH2)2NHMe2][B5O6(OH)4]2 (1), [ Et2NH( )2NHEt2][B5O6(OH)4]2 (2), [Et2NH2][B5O6(OH)4] (3), [Me2NH2][B5O6(OH)4] (4), [Me2NH( ) )3NHMe2][B5O6(OH)4]2(5),[Et2NH( )3NHEt2][B5O6(OH)4]2(6),[Me3N CH= ][B5O6(OH)4](7), [Me3N( )3NMe3][B5O6(OH)4]2.0.5H2O (8
表征谱图
-
氢谱1HNMR
-
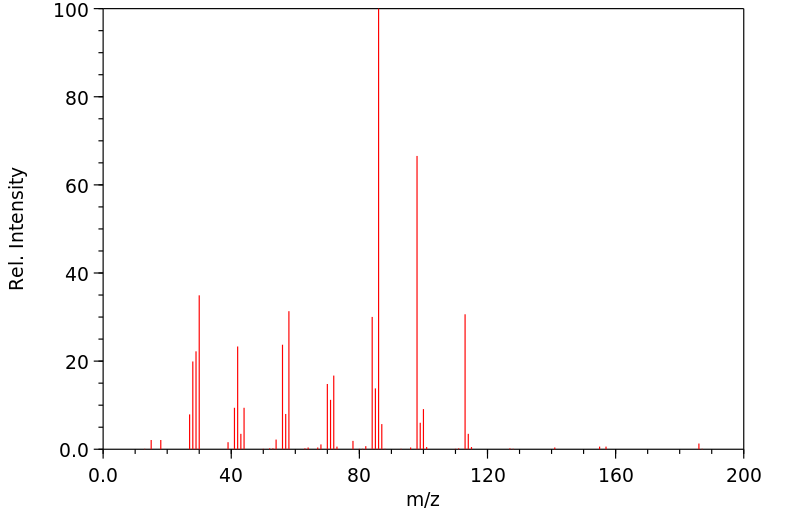
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷