2-Chlor-9-methyl-phenazin | 29453-82-5
中文名称
——
中文别名
——
英文名称
2-Chlor-9-methyl-phenazin
英文别名
8-chloro-1-methylphenazine;8-Chlor-1-methyl-phenazin
CAS
29453-82-5
化学式
C13H9ClN2
mdl
——
分子量
228.681
InChiKey
BFIBVWUZFNYPKE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of 1- and 2-Morpholinophenazine Derivatives摘要:已经通过各种卤代苯并呲嗪衍生物与吗啉的反应制备了一些新的吗啉苯并呲嗪。研究发现,在卤代苯并呲嗪-N-氧化物中,用吗啉替换卤素原子时伴随着氧化物基团的去除,并且在这些反应中没有形成吗啉苯并呲嗪-N-氧化物。DOI:10.1246/bcsj.42.506
-
作为产物:描述:N-(5-chloro-2-nitrosophenyl)-N-(2-methylphenyl)amine 在 N,O-双三甲硅基乙酰胺 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 48.0h, 以93%的产率得到2-Chlor-9-methyl-phenazin参考文献:名称:N-芳基-2-亚硝基苯胺作为中间体,由硝基芳烃合成取代的吩嗪摘要:可从硝基芳烃与苯胺阴离子上反应得到的N-芳基-2-亚硝基苯胺经过环化以提供取代的吩嗪。该反应通过在甲醇中的碳酸钾,在非质子溶剂中的N,O-双(三甲基甲硅烷基)乙酰胺(BSA)和乙酸来促进。从合适的硝基芳烃-苯胺对开始,合成1-花青素的前体1-甲氧基吩嗪就说明了该方法。DOI:10.1016/j.tetlet.2011.09.113
表征谱图
-
氢谱1HNMR
-
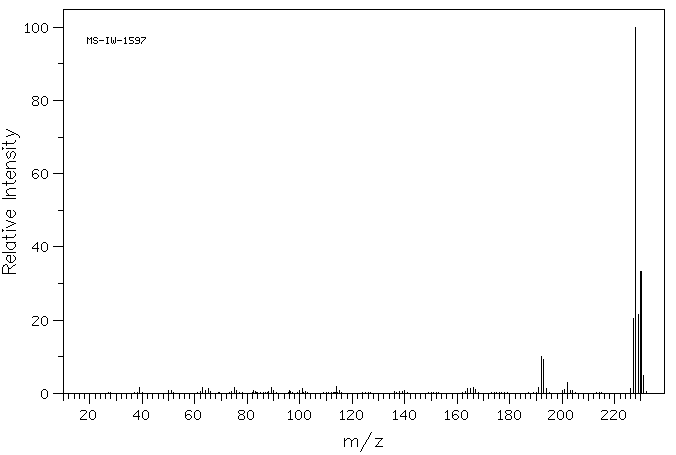
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮