(C-methylsulfanylcarbonimidoyl)azanium;sulfate
中文名称
——
中文别名
——
英文名称
(C-methylsulfanylcarbonimidoyl)azanium;sulfate
英文别名
——
CAS
——
化学式
C4H14N4O4S3
mdl
MFCD00013055
分子量
278.4
InChiKey
BZZXQZOBAUXLHZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.41
-
重原子数:15
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:233
-
氢给体数:6
-
氢受体数:8
反应信息
-
作为反应物:参考文献:名称:Method for the treatment of arrhythmia摘要:一种通过给予氨基脲类化合物治疗心律失常的方法。公开号:US04178387A1
文献信息
-
Method for treatment of gastrointestinal secretory and ulcerogenic申请人:William H. Rorer, Inc.公开号:US04169155A1公开(公告)日:1979-09-25A new class of chemical compounds and their process of preparation is described. These compounds have valuable properties as anti-secretory, anti-spasmodic, anti-ulcerogenic and anti-diarrheal agents.描述了一种新型化合物及其制备过程。这些化合物具有抗分泌、抗痉挛、抗溃疡和抗腹泻药物的有价值性能。
-
Amidinourea local anesthetics
-
Sulfonamide derivatives, their production and use申请人:——公开号:US20020193382A1公开(公告)日:2002-12-19The present invention is to provide a compound or a salt thereof represented by the formula: 1 wherein R 1 is an optionally substituted hydrocarbon group or an optionally substituted heterocyclic group, the ring A is an optionally substituted divalent nitrogen-containing heterocyclic group, X′ is an optionally substituted alkylene chain, Y is an optionally substituted divalent cyclic group, X is a chemical bond or an optionally substituted alkylene chain, and Z is (1)an optionally substituted amino group, (2) an optionally substituted imidoyl group or (3) an optionally substituted nitrogen-containing heterocyclic group, or a pro-drug thereof, which have activated coagulation factor X inhibitory activity and which is useful as anti-coagulant.
-
Method of inhibiting histamine activity with guanidine compounds
-
(Substituted aralkyl) heterocyclic compounds申请人:Imperial Chemical Industries plc公开号:US04935437A1公开(公告)日:1990-06-19A (substituted-aralkyl)heterocyclic compound of the formula I ##STR1## wherein R.sup.1 is an azido, carbamoyl, cyano, formyl, hydroxy or nitro radical, a 1-6C 1-hydroxyalkyl, alkoxy, alkylcarbamoyl, alkylthio, alkylsulphinyl or alkylsulphonyl radical, a 2-cyanoethyl radical, optionally bearing one to four 1-6C alkyl substituents, or a 2-6C alkanoyl, halogenoalkanoyl, alkanoyloxy, alkanoylamino, dialkylcarbamoyl or alkoxycarbonyl radical; R.sup.2 and R.sup.3, which may be the same or different, are each a hydrogen atom, a 1-6C alkyl, dueterioalkyl or halogenoalkyl radical, or a phenyl or phenyl(1-6C alkyl) radical, in each of which the phenyl may optionally bear one or more substituents; or R.sup.2 and R.sup.3, together with the carbon atom to which they are attached, may form a 3- to 6-membered ring; or R.sup.1 R.sup.2 R.sup.3 C- is a 1,1-dicyanoethyl or trifluoromethylsulphonyl radical; R.sup.4 is a hydrogen or halogen atom, a cyano or nitro radical or a 1-6C alkyl or halogenoalkyl radical; R.sup.5 has any of the values defined above for the group R.sup.1 R.sup.2 R.sup.3 C but is not necessarily the same as R.sup.1 R.sup.2 R.sup.3 C, or has any of the values defined above for R.sup.4 but is not necesarily the same as R.sup.4, or is a carbamoyl, 1-pyrrolidinyl-carbonyl, piperidinocarbonyl, morpholinocarbonyl or nitro radical, a 1-6C alkoxy or halogenoalkoxy radical or a 2-6C alkanoyl or alkoxy-carbonyl radical; A is a methylene or ethylene radical optionally bearing one or more substituents selected from deuterium and halogen atoms, carbamoyl, cyano and hydroxy radicals, 1-6C alkyl and alkoxy radicals, and 2-6C alkanoyloxy radicals provided that when A is linked to R.sup.6 through a nitrogen atom thereof, it may not bear a hydroxy, alkoxy or alkanoyloxy substituent on the carbon atom adjacent to such nitrogen atoms; and R.sup.6 is a 1H-1,2,4-triazol-1-yl, 4H-1,2,4-triazol-4-yl, 1H-imidazol-1-yl, 5-cyano-1H-imidazol-1-yl, 3-pyridyl or 5-pyrimidinyl radical, or a 1H-imidazol-1-yl radical, bearing at the 5-position thereof a 1-6C alkyl substituent which is itself optionally substituted by one or more carbamoyl, cyano, hydroxy or 2-6C alkoxycarbonyl radicals; and provided that when R.sup.2, R.sup.3, R.sup.4 and R.sup.5 are hydrogen, A is a methylene radical and R.sup.6 is a 3-pyridyl radical, R.sup.1 is not a cyano, hydroxy or hydroxymethyl radical, and when R.sup.1 is a hydroxy radical, R.sup.3, R.sup.4 and R.sup.5 are hydrogen, A is a methylene radical and R.sup.6 is 3-pyridyl, R.sup.2 is not a methyl or a 2-chloro-1-methylethyl radical, and provided that when R.sup.1 is a methoxycarbonyl radical, R.sup.2, R.sup.3, R.sup.4 and R.sup.5 are hydrogen and A is a methylene radical, R.sub.1 is not a 1H-imidazol-1-yl radical; and the pharmaceutically acceptable acid addition salts thereof.化合物I的结构式如下:##STR1## 其中R.sup.1为偶氮基、氨基甲酰基、氰基、甲酰基、羟基或硝基基团、1-6C 1-羟基烷基、烷氧基、烷基氨甲酰基、烷基硫醇基、烷基亚磺酰基或烷基磺酰基基团、2-氰乙基基团,可选地带有1-4个1-6C烷基取代基,或为2-6C脂肪酰基、卤代脂肪酰基、脂肪酰氧基、脂肪酰胺基、二烷基氨基甲酰基或烷氧羰基基团;R.sup.2和R.sup.3,可以相同或不同,分别为氢原子、1-6C烷基、氘代烷基或卤代烷基基团,或苯基或苯基(1-6C烷基)基团,在其中苯基可以可选地带有一个或多个取代基;或R.sup.2和R.sup.3,连同它们所连接的碳原子,可以形成一个3-至6-成员环;或R.sup.1 R.sup.2 R.sup.3 C-为1,1-二氰基乙基或三氟甲基磺酰基基团;R.sup.4为氢或卤素原子、氰基或硝基基团或1-6C烷基或卤代烷基基团;R.sup.5具有上述R.sup.1 R.sup.2 R.sup.3 C基团定义的任何值,但不一定与R.sup.1 R.sup.2 R.sup.3 C相同,或具有上述R.sup.4定义的任何值,但不一定与R.sup.4相同,或为氨基甲酰基、1-吡咯啉基甲酰基、哌啶基甲酰基、吗啉基甲酰基或硝基基团、1-6C烷氧基或卤代烷氧基基团或2-6C脂肪酰基或烷氧羰基基团;A为甲基或乙基基团,可选地带有一个或多个取代基,所选取代基包括氘和卤素原子、氨基甲酰基、氰基和羟基基团、1-6C烷基和烷氧基、以及2-6C脂肪酰氧基取代基,但当A通过其中一个氮原子与R.sup.6连接时,它不能在相邻的碳原子上带有羟基、烷氧基或烷基羧酸酯取代基;而R.sup.6为1H-1,2,4-三唑-1-基、4H-1,2,4-三唑-4-基、1H-咪唑-1-基、5-氰基-1H-咪唑-1-基、3-吡啶基或5-嘧啶基基团,或1H-咪唑-1-基,其在5-位置带有一个1-6C烷基取代基,该烷基本身可选地被一个或多个氨基甲酰基、氰基、羟基或2-6C烷氧羰基基团取代;并且当R.sup.2、R.sup.3、R.sup.4和R.sup.5为氢时,A为甲基基团,而R.sup.6为3-吡啶基基团时,R.sup.1不是氰基、羟基或羟甲基基团,当R.sup.1为羟基基团时,R.sup.3、R.sup.4和R.sup.5为氢,A为甲基基团,而R.sup.6为3-吡啶基基团时,R.sup.2不是甲基或2-氯-1-甲基乙基基团;当R.sup.1为甲氧羰基基团时,R.sup.2、R.sup.3、R.sup.4和R.sup.5为氢,A为甲基基团时,R.sup.1不是1H-咪唑-1-基;以及其药学上可接受的酸盐。
表征谱图
-
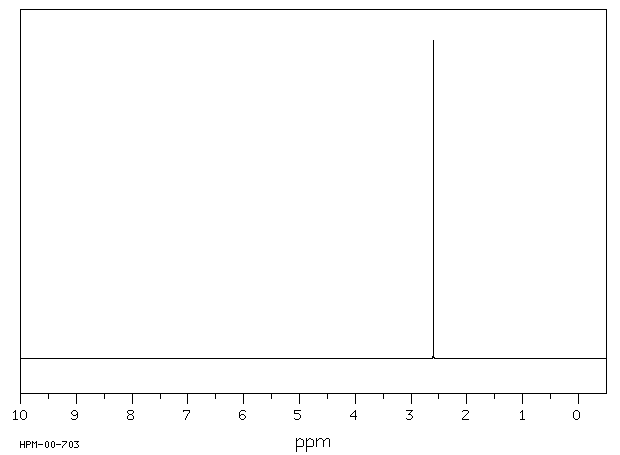
氢谱1HNMR
-
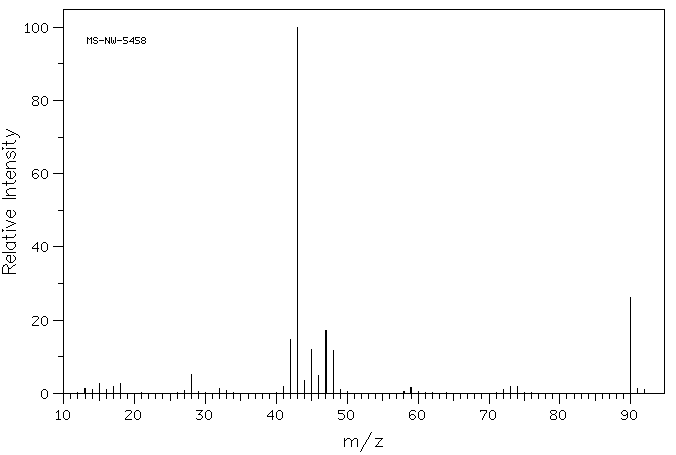
质谱MS
-
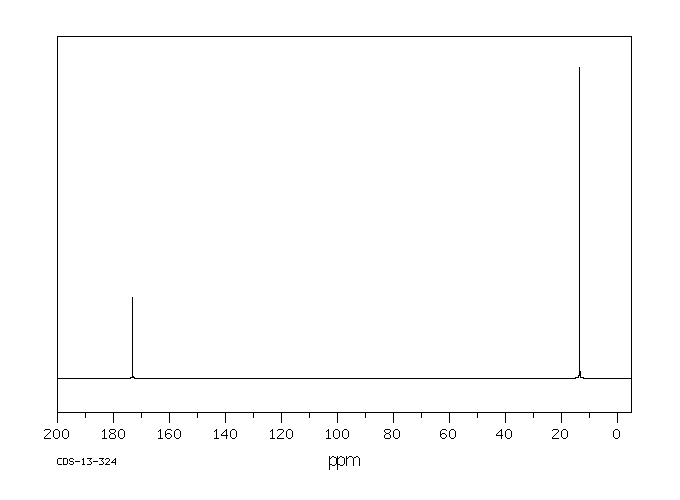
碳谱13CNMR
-
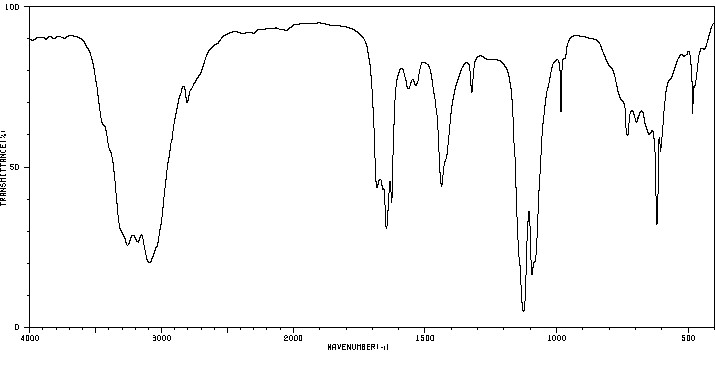
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-BOC-5-甲基-1,2,3-氧杂噻唑烷-2,2-二氧化物
(4S)-4-叔丁基-1,2,3-氧杂噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S)-4-i-丙基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R)-4-叔丁基-1,2,3-氧杂噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
鲸蜡硬脂醇硫酸酯钠盐
高香草酸硫酸盐
鞘氨醇
非氯双链季铵盐
靛草素
阿维巴坦中间体5
间硝基苯基甲基硫酸盐
镧硫酸二乙酯
镁己基硫酸盐
铵硫酸癸酯盐
铵硫酸甲酯盐
铵己基硫酸盐
铵二十烷基硫酸盐
铵二十二烷基硫酸酯盐
铵2,3-二溴丙基硫酸盐
铝十二烷基硫酸盐
钾十八烷基硫酸酯盐
钾二十二烷基硫酸酯盐
钾p-氨基苯基硫酸盐
钾(4-硝基苯基)硫酸盐
钠硫酸丙酯盐
钠癸烷-2-基硫酸盐
钠氨基甲酰(羟基)氨基磺酸
钠二十六烷基硫酸酯盐
钠乙酰基硫酸盐
钠[(2-羟基乙基)锍二基]二-2,1-乙二基二硫酸盐
钠2-己氧乙基硫酸盐
钙十三烷基硫酸盐
钙二(甲基氨基磺酸)
酸性甘油-1,3-二硫酸盐
酸式硫酸三乙基锡
酚酞二硫酸酯钾盐
酒石酸去甲肾上腺素杂质3钠盐
邻苯二酚-4-磺酸铵
辛基硫酸钠
辛基硫酸酯钾盐
辛基硫酸氢酯
辛基-2-硫酸酯
莎拉西娅根茎提取物
草莓酸
苯肾上腺素O-芳基硫酸盐
苯肼,硫酸盐
苯氧基磺酰基7-甲基辛酸酯
苯基硫酸钠盐
苯基2,2,2-三氯乙基硫酸盐
脲硫酸盐(1:1)