3-methyl-1H,5H-naphtho[1,8-ef][1,3]dithiocine | 115029-69-1
中文名称
——
中文别名
——
英文名称
3-methyl-1H,5H-naphtho[1,8-ef][1,3]dithiocine
英文别名
3-methyl-1H,5H-naphto<1,8-ef><1,3>dithiocin;3-methyl-1H,5H-naphtho<1,8-ef><1,3>dithiocin;3-Methyl-1H,5H-naphtho(1,8-ef)(1,3)dithiocin;12-methyl-11,13-dithiatricyclo[7.5.1.05,15]pentadeca-1,3,5(15),6,8-pentaene
CAS
115029-69-1
化学式
C14H14S2
mdl
——
分子量
246.397
InChiKey
HIKIVDWONDOWPT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 [8-(硫烷基甲基)萘-1-基]甲硫醇 1,8-bis(mercaptomethyl)naphthalene 60948-99-4 C12H12S2 220.359
反应信息
-
作为反应物:描述:3-methyl-1H,5H-naphtho[1,8-ef][1,3]dithiocine 在 间氯过氧苯甲酸 作用下, 以 二氯甲烷 为溶剂, 以44%的产率得到3-methyl-1H,5H-naphtho[1,8-ef][1,3]dithiocine 2-oxide参考文献:名称:Synthesis and Stereochemistry of 1H,5H-Naphtho[1,8-ef][1,3]dithiocine 2-Oxides摘要:3-取代的 1H、5H-萘并[1,8-ef][1,3]二硫杂环丁烷(R = H、Me、Ph、t-Bu)被间氯过氧苯甲酸氧化成反式构型(R≠H)的相应 2-氧化物。根据 1H 和 13C NMR 数据(包括 NOESY 实验),二取代化合物在室温下呈舟形构象,C3 上的取代基和 S2 上的氧原子呈赤道取向。而 C3 上没有取代基的化合物则产生了轴向和赤道亚砜氧原子比例为 83:17 的舟形构象混合物。DOI:10.1007/s11178-005-0297-5
-
作为产物:描述:1,8-双(溴甲基)萘 在 三氟化硼乙醚 氢氧化钾 作用下, 以 乙醇 、 氯仿 、 水 为溶剂, 反应 5.0h, 生成 3-methyl-1H,5H-naphtho[1,8-ef][1,3]dithiocine参考文献:名称:1H,5H-Naphtho[1,8-ef][1,3]dithiocin 系统的合成摘要:由 1,8-萘二甲酸酐分四步制备了名为新的全环萘体系,总产率为 35-78%。合成中的关键步骤采用 1,8-双(巯基甲基)萘与羰基化合物的二硫缩醛环化反应,使用三氟化硼醚合物作为催化剂。DOI:10.1246/bcsj.62.3024
文献信息
-
KAMADA, TOSHIHIRO;GAMA, YASUO;WASADA, NOBUHIDE, BULL. CHEM. SOC. JAP., 62,(1989) N, C. 3024-3025作者:KAMADA, TOSHIHIRO、GAMA, YASUO、WASADA, NOBUHIDEDOI:——日期:——
-
KLIMOVITSKIJ, E. N.;SERGEEVA, G. N.;ILYASOV, K. A.;LATYPOV, SH. K.;KLOCHK+, ZH. OBSHCH. XIMII, 57,(1987) N 10, 2207-2214作者:KLIMOVITSKIJ, E. N.、SERGEEVA, G. N.、ILYASOV, K. A.、LATYPOV, SH. K.、KLOCHK+DOI:——日期:——
表征谱图
-
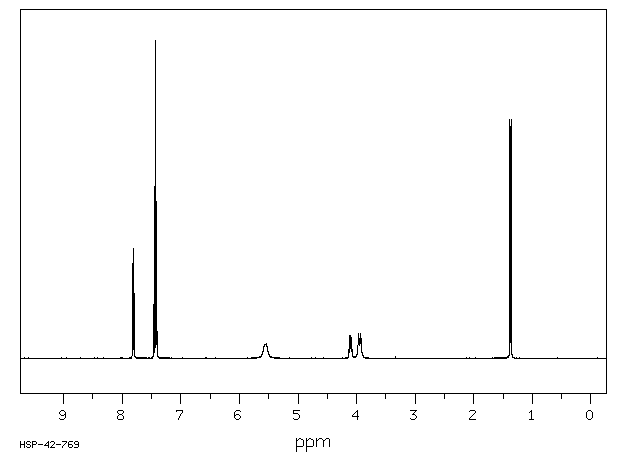
氢谱1HNMR
-
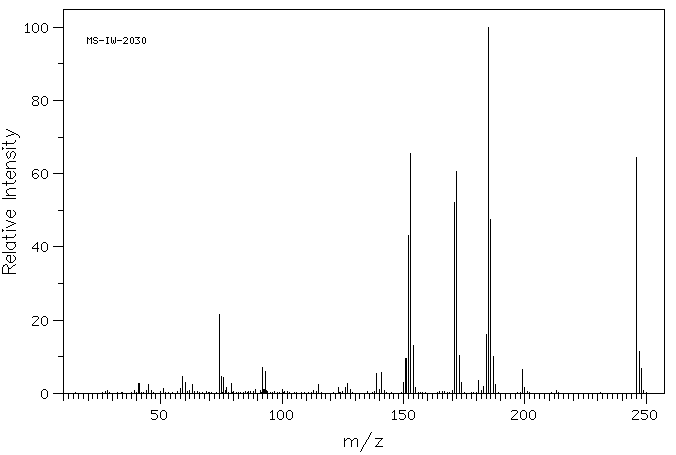
质谱MS
-
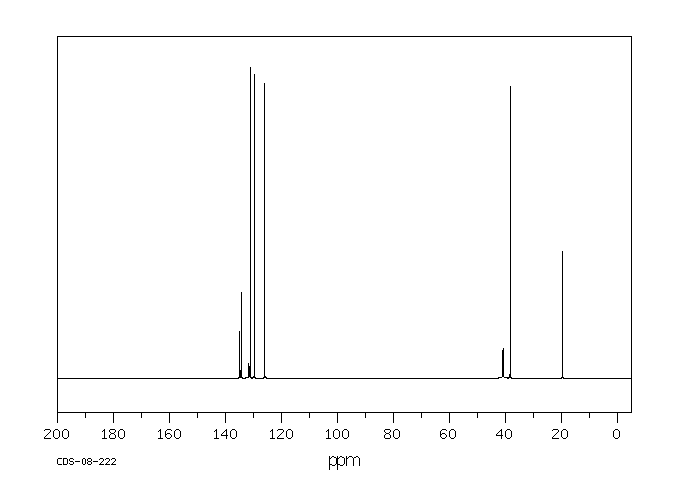
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮