2-phenyl-1,2,3,4-tetrahydronaphthalen-1-ol | 93433-58-0
中文名称
——
中文别名
——
英文名称
2-phenyl-1,2,3,4-tetrahydronaphthalen-1-ol
英文别名
trans-2-Phenyl-1,2,3,4-tetrahydro-1-naphthol
CAS
93433-58-0
化学式
C16H16O
mdl
——
分子量
224.302
InChiKey
OKDIYKQVCBYZIJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
反应信息
-
作为反应物:描述:参考文献:名称:Campbell; Kidd, Journal of the Chemical Society, 1954, p. 2154摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 lithium aluminium tetrahydride 、 乙醚 作用下, 生成 2-phenyl-1,2,3,4-tetrahydronaphthalen-1-ol参考文献:名称:Campbell; Kidd, Journal of the Chemical Society, 1954, p. 2154摘要:DOI:
文献信息
-
Synthetic route for the preparation of substituted 2-phenyl-1,2,3,4-tetrahydronaphthalene-1-ols申请人:LEK Pharmaceuticals d.d.公开号:EP2644603A1公开(公告)日:2013-10-02The present invention relates in general to the field of organic chemistry and in particular to the preparation of 2-phenyl-1,2,3,4-tetrahydronaphthalene-1-ol substituted with a protected hydroxyl group. These compounds can be used as intermediates in the synthesis of pharmaceutically active agents such as lasofoxifene or derivatives thereof.
-
Copper-catalyzed asymmetric hydrogenation of 2-substituted ketones <i>via</i> dynamic kinetic resolution作者:Olga V. Zatolochnaya、Sonia Rodríguez、Yongda Zhang、Kendricks S. Lao、Sergei Tcyrulnikov、Guisheng Li、Xiao-Jun Wang、Bo Qu、Soumik Biswas、Hari P. R. Mangunuru、Daniel Rivalti、Joshua D. Sieber、Jean-Nicolas Desrosiers、Joyce C. Leung、Nelu Grinberg、Heewon Lee、Nizar Haddad、Nathan K. Yee、Jinhua J. Song、Marisa C. Kozlowski、Chris H. SenanayakeDOI:10.1039/c8sc00434j日期:——A new class of tunable heterophosphole dimeric ligands have been designed and synthesized. These ligands have enabled the first examples of Cu-catalyzed hydrogenation of 2-substituted-1-tetralones and related heteroaryl ketones via dynamic kinetic resolution, simultaneously creating two contiguous stereogenic centers with up to >99 : 1 dr and 98 : 2 er. The ligand-Cu complexes were isolated and characterized
-
RuPHOX–Ru catalyzed asymmetric hydrogenation of α-substituted tetralones <i>via</i> a dynamic kinetic resolution作者:Jingjing Li、Jianxun Ye、Jiayu Zhou、Jing Li、Delong Liu、Wanbin ZhangDOI:10.1039/d2cc01193j日期:——The efficient RuPHOX–Ru catalyzed asymmetric hydrogenation of α-substituted tetralones via a dynamic kinetic resolution has been achieved for the synthesis of chiral tetrahydronaphthols. The mechanism study indicated that hydrogenation with H2 gas, rather than transfer hydrogenation with EtOH solvent as the hydrogen source, predominates in the reaction pathway.
-
Diastereoselective Friedel–Crafts Alkylation of Hydronaphthalenes作者:Jennifer Tsoung、Katja Krämer、Adam Zajdlik、Clemence Liébert、Mark LautensDOI:10.1021/jo201781x日期:2011.11.4An efficient and versatile synthesis of chiral tetralins has been developed using both inter- and intra-molecular Friedel Crafts alkylation as a key step. The readily available hydronaphthalene substrates were prepared via a highly enantioselective metal-catalyzed ring opening of meso-oxabicyclic alkenes followed by hydrogenation. A wide variety of complex tetracyclic compounds have been isolated with high levels of regio-, diastereo-, and enantioselectivity.
-
Synthetic Estrogens, Implantation Inhibitors, and Hypocholesterolemic Agents. I. Tetrahydronaphthalene Series作者:W. L. Bencze、L. I. Barsky、R. W. J. Carney、A. A. Renzi、George. deStevensDOI:10.1021/jm00314a002日期:1967.3
表征谱图
-
氢谱1HNMR
-
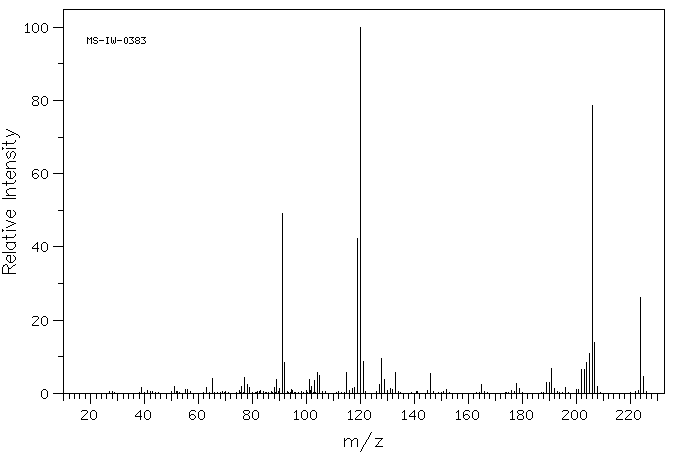
质谱MS
-
碳谱13CNMR
-
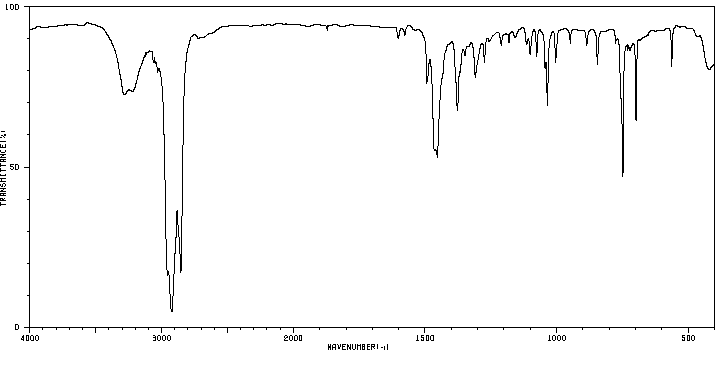
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮