2-碘-4-甲基-6-硝基苯酚 | 69492-91-7
中文名称
2-碘-4-甲基-6-硝基苯酚
中文别名
——
英文名称
2-iodo-4-methyl-6-nitrophenol
英文别名
6-Iod-4-methyl-2-nitrophenol;2-Jod-4-methyl-6-nitro-phenol;6-Jod-2-nitro-p-kresol;5-Jod-3-nitro-4-oxy-toluol
CAS
69492-91-7
化学式
C7H6INO3
mdl
MFCD00060537
分子量
279.034
InChiKey
ZGSKNXKQYFZYAW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:82-84°C
-
沸点:254.4±35.0 °C(Predicted)
-
密度:2.021±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:66
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2908999090
-
安全说明:S26,S36/37/39
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-硝基-4-甲苯酚 4-methyl-2-nitrophenol 119-33-5 C7H7NO3 153.137
反应信息
-
作为反应物:描述:2-碘-4-甲基-6-硝基苯酚 在 吗啉 、 manganese(IV) oxide 、 copper(l) iodide 、 palladium 10% on activated carbon 、 三苯基膦 作用下, 以 乙醇 、 二氯甲烷 、 水 为溶剂, 反应 12.0h, 生成 (E)-4-bromo-N'-[(5-methyl-7-nitrobenzofuran-2-yl)methylene]benzohydrazide参考文献:名称:Synthesis of hydrazone derivatives of benzofuran and their antibacterial and antifungal activity摘要:A series of benzofuran hydrazones 6a-6n were synthesized from benzofuran aldehyde and substituted aromatic hydrazides 5a-5n. Structures of all compounds were confimed by IR, H-1 and C-13 NMR, and Mass spectral data. These compounds were evaluated for their antibacterial activity against gram-negative bacteria (Escherichia coli, -ve), gram-positive bacteria (Bacillus Subtillis, +ve), and antifungal activity against Candida albicans. All compounds demonstrated considerable activity against bacteria and fungi.DOI:10.1134/s1070363217090183
-
作为产物:描述:2-硝基-4-甲苯酚 在 碳酸氢钠 、 N,N,N-trimethylbenzenemethanaminium dichloroiodate 作用下, 以 甲醇 、 二氯甲烷 为溶剂, 反应 6.0h, 以95%的产率得到2-碘-4-甲基-6-硝基苯酚参考文献:名称:使用苄基三甲基二氯碘酸铵(1-)碘化苯酚摘要:在 CaCO3 或 NaHCO3 存在下,酚类与二氯碘酸苄基三甲基铵 (1-) 在二氯甲烷 - 甲醇中在室温下反应几个小时,以良好的产率得到碘酚。DOI:10.1246/cl.1987.2109
文献信息
-
Palladium catalyzed ring opening of furans as a route to α,β-unsaturated aldehydes作者:Laurent El Kaïm、Laurence Grimaud、Simon WagschalDOI:10.1039/c0cc04164e日期:——Furans may be ring opened via pallado-catalyzed reactions leading to alpha,beta-unsaturated aldehydes and ketones tethered to indole and isoquinoline moieties. Besides their synthetic interest, these fragmentations bring interesting elements into the discussion around the reaction mechanisms involved in palladium C-H activations of electron-rich heterocycles.
-
Palladium-Catalyzed Ring Opening of Aminocyclopropyl Ugi Adducts作者:Laurent El Kaïm、Laurence Grimaud、Aurélie Dos Santos、Romain RamozziDOI:10.1055/s-0031-1290312日期:2012.2The ring opening of aminocyclopropanes triggered by activation with an intramolecular arylpalladium(II) iodide complex is an interesting strategy for the synthesis of nitrogen heterocycles and a valuable Ugi postcondensation-type transformation. Six- and seven-membered-ring cyclic enamines may be obtained. aminocyclopropanes - ring opening - palladium - Ugi - Ugi-Smiles
-
Datta; Prosad, Journal of the American Chemical Society, 1917, vol. 39, p. 452作者:Datta、ProsadDOI:——日期:——
-
Jones; Richardson, Journal of the Chemical Society, 1953, p. 713作者:Jones、RichardsonDOI:——日期:——
-
Gaude, Didier; Gellon, Gisele; Goaller, Raymond Le, Canadian Journal of Chemistry, 1989, vol. 67, p. 104 - 108作者:Gaude, Didier、Gellon, Gisele、Goaller, Raymond Le、Pierre, Jean-LouisDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
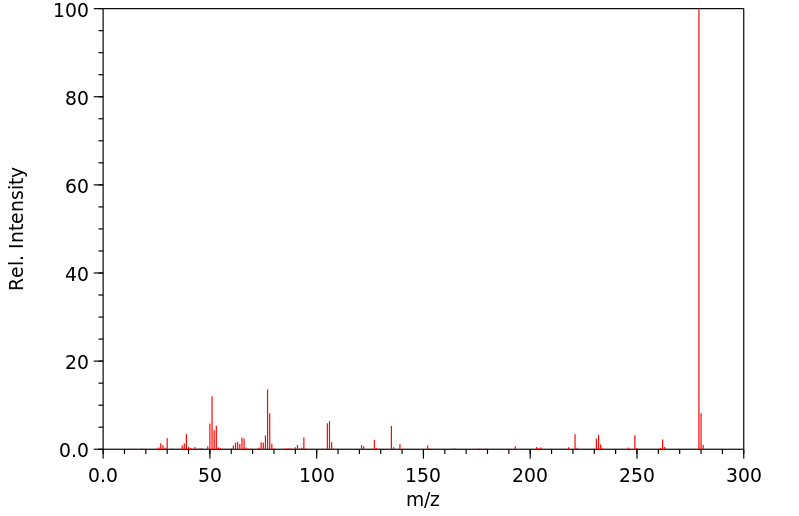
质谱MS
-
碳谱13CNMR
-
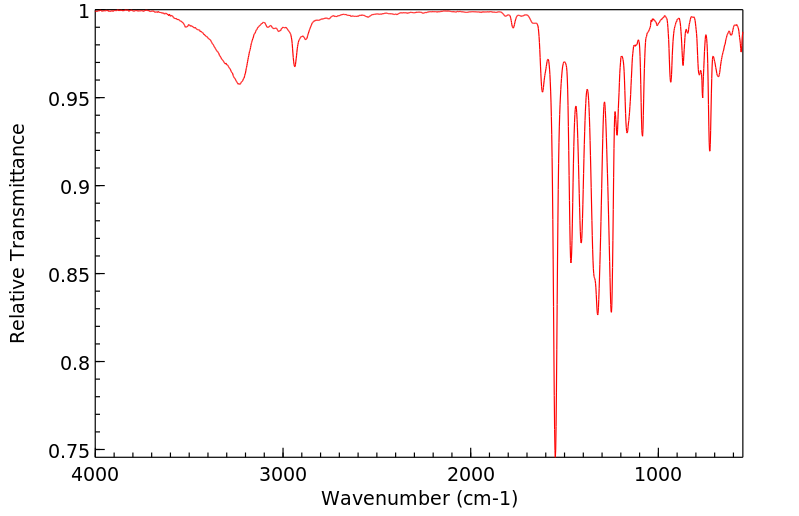
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚