2-甲基-3-噻吩甲酸乙酯 | 19432-66-7
中文名称
2-甲基-3-噻吩甲酸乙酯
中文别名
2-甲基噻吩-3-羧酸乙酯
英文名称
ethyl 2-methyl-thiophene-3-carboxylate
英文别名
ethyl 2-methyl-3-thiophenecarboxylate;ethyl 2-methylthiophene-3-carboxylate;2-methyl-3-thiophenecarboxylic acid ethyl ester
CAS
19432-66-7
化学式
C8H10O2S
mdl
——
分子量
170.232
InChiKey
GNOBQWZOPPOTMC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:222.0±20.0 °C(Predicted)
-
密度:1.14g/ml
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:54.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
-
危险性防范说明:P264,P270,P301+P312,P330
-
危险性描述:H302
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Ethyl 2-methylthiophene-3-carboxylate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Ethyl 2-methylthiophene-3-carboxylate
CAS number: 19432-66-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H10O2S
Molecular weight: 170.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Ethyl 2-methylthiophene-3-carboxylate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Ethyl 2-methylthiophene-3-carboxylate
CAS number: 19432-66-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H10O2S
Molecular weight: 170.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-3-噻吩甲酸 2-methylthiophene-3-carboxylic acid 1918-78-1 C6H6O2S 142.178 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-3-噻吩甲酸 2-methylthiophene-3-carboxylic acid 1918-78-1 C6H6O2S 142.178
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and antimicrobial activity of some netropsin analogues摘要:合成了九种新型lexitropsin,通过三种不同的二羧酸(9,10-二氢-2,7-菲二羧酸;[(3-{[(羧甲基)氨基]羰基}苯甲酰)氨基]乙酸和吲哚-2,5-二羧酸)将两个类似netropsin的部分连接在一起。通过使用N-异戊基吡咯、5-甲基噻吩或5-异丙基噻唑异构体构建单元替代通常的N-甲基吡咯,对netropsin残基进行修饰。将这些化合物分别针对五种革兰阳性菌:金黄色葡萄球菌、肠球菌、耐甲氧西林金黄色葡萄球菌、克雷伯氏菌和偶然分枝杆菌,三种革兰阴性菌:气单胞菌、变形杆菌、大肠杆菌,以及三种真菌:黑曲霉、白念珠菌和安氏曲霉进行了测试。其中一些化合物对微生物的生长表现出显著的抑制作用。DOI:10.1039/b408386p
-
作为产物:描述:(1-ethoxycarbonylcyclopropyl)triphenylphosphonium tetrafluoroborate 在 2,3-dichloro-4,5-dicyanoquinone 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 反应 74.0h, 生成 2-甲基-3-噻吩甲酸乙酯参考文献:名称:Chatterjee, Pallab; Murphy, Patrick J.; Pepe, Rosanna, Journal of the Chemical Society. Perkin transactions I, 1994, # 17, p. 2403 - 2406摘要:DOI:
文献信息
-
Phosphorylamides, their preparation and use申请人:Takeda Chemical Industries, Ltd.公开号:US05840917A1公开(公告)日:1998-11-24A phosphorylamide derivative represented by the general formula (I): ##STR1## wherein R represents an amino group that may be substituted, or a salt thereof, possesses potent antibacterial activity against Helicobacter bacterium, especially Helicobacter pylori, and is useful for prevention or treatment of digestive diseases caused by Helicobacter bacterium, solely or in combination with an antacid or an acid secretion inhibitor.
-
Convenient Method for the Preparation of the 2-Methyl Thiophen-3-yl Magnesium Bromide Lithium Chloride Complex and Its Application to the Synthesis of 3-Substituted 2-Methylthiophenes作者:Masakazu Kogami、Nobuhide WatanabeDOI:10.1080/00397911.2011.606391日期:2013.1.1formation of the Grignard reagent from inactive 3-bromo-2-methylthiophene (1) and commercial magnesium metal. Based on this finding, a convenient and potentially scalable preparation of ethyl 2-methylthiophene-3-carboxylate (3) was achieved. In addition, this process has been found to provide a new, general approach to 3-substituted 2-methylthiophenes. GRAPHICAL ABSTRACT
-
[EN] NOVEL TRICYCLIC COMPOUNDS<br/>[FR] NOUVEAUX COMPOSÉS TRICYCLIQUES申请人:JACOBIO BETA PHARMACEUTICALS CO LTD公开号:WO2019080941A1公开(公告)日:2019-05-02This invention relates to certain novel tricyclic compounds as bromodomain and extra-terminal (BET) inhibitors, their synthesis and their use for treating diseases. More particularly, this invention is directed to fused heterocyclic derivatives useful as inhibitors of BET, methods for producing such compounds and methods for treating diseases and conditions wherein inhibition of one or more BET bromodomains provides a benefit.
-
[EN] BISARYL HETEROCYCLES AS NRF2 ACTI<br/>[FR] HÉTÉROCYCLES BISARYLE EN TANT QUE NRF2 ACTI申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2018109643A1公开(公告)日:2018-06-21The present invention relates to bisaryl heterocycle compounds, methods of making them, pharmaceutical compositions containing them and their use as NRF2 activators. In particular, the invention relates to bisaryl heterocycles of Formula (I),
-
Practical Preparation of Ethyl 2-Methylthiophene-3-carboxylate作者:Masakazu Kogami、Nobuhide WatanabeDOI:10.1248/cpb.59.797日期:——A safe and efficient process for the preparation of ethyl 2-methylthiophene-3-carboxylate (5) was devised. This process provides several advantages over the precedents, involving operational simplicity, avoidance of the use of strong bases such as n-butyllithium and application of noncryogenic conditions, and enabled to prepare 5 in 52% overall yield from commercially available 2-methylthiophene on
表征谱图
-
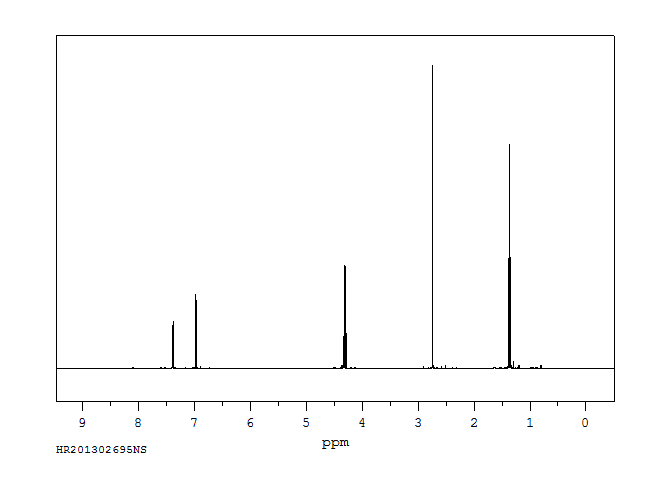
氢谱1HNMR
-
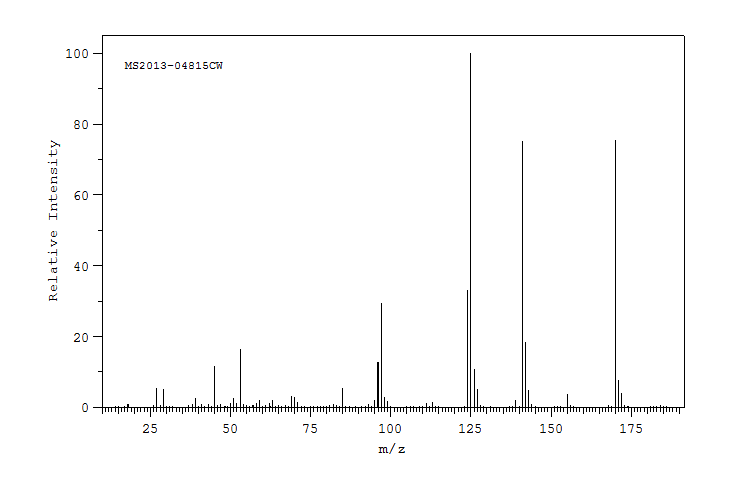
质谱MS
-
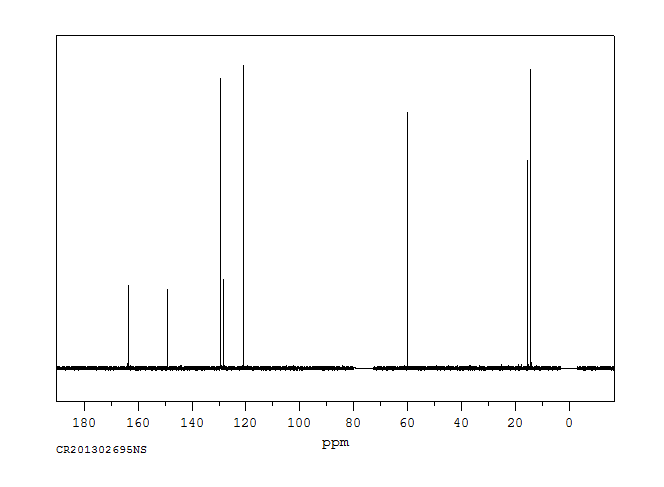
碳谱13CNMR
-
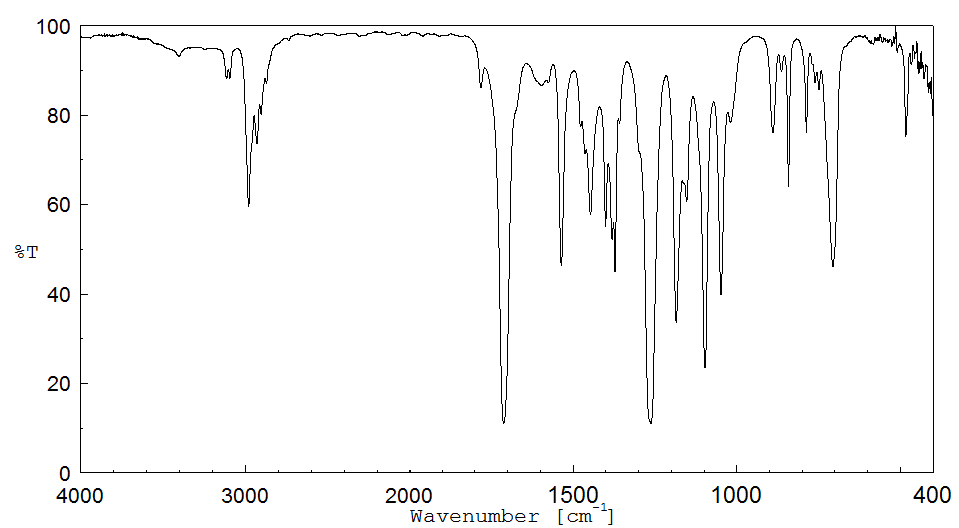
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯