磷酸脲 | 4861-19-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:116-118 °C (dec.)(lit.)
-
密度:1.77[at 20℃]
-
溶解度:在水中的溶解度50 mg/mL,澄清
-
LogP:-1.73 at 20℃
-
物理描述:DryPowder; Liquid
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-1.9
-
重原子数:9
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:147
-
氢给体数:5
-
氢受体数:5
安全信息
-
危险品标志:C
-
安全说明:S22,S24/25
-
危险类别码:R34
-
WGK Germany:1
-
危险品运输编号:UN 3261 8 / PGII
-
海关编码:3105590000
SDS
Section 1. Chemical Product and Company Identification
Urea Phosphate
Common Name/
Trade Name
Manufacturer
Commercial Name(s)
Synonym
Chemical Name
Chemical Family
Urea Phosphate
Section 4. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at
least 15 minutes. Get medical attention if irritation occurs.
Skin Contact Wash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops.
Serious Skin Contact Not available.
Inhalation If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get
medical attention.
Serious Inhalation Not available.
Ingestion Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an
unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight
clothing such as a collar, tie, belt or waistband.
Serious Ingestion Not available.
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
Flash Points Not available.
Flammable Limits Not available.
Products of Combustion These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...), phosphates.
Fire Hazards in Presence of Slightly flammable to flammable in presence of heat.
Various Substances
Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
of Various Substances
SMALL FIRE: Use DRY chemical powder.
Fire Fighting Media
and Instructions LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
As with most organic solids, fire is possible at elevated temperatures
Special Remarks on
Fire Hazards
Special Remarks on Explosion Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust
Hazards explosion hazard.
Section 6. Accidental Release Measures
Small Spill Use appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by
spreading water on the contaminated surface and dispose of according to local and regional authority
requirements.
Large Spill Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water
on the contaminated surface and allow to evacuate through the sanitary system.
Urea Phosphate
Section 7. Handling and Storage
Precautions Keep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not
breathe dust. Keep away from incompatibles such as oxidizing agents.
Storage Keep container tightly closed. Keep container in a cool, well-ventilated area.
Section 8. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection
Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Use a
dust respirator if ventilation is inadequate and dust is visible. Gloves.
Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used
a Large Spill to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist
BEFORE handling this product.
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Physical state and appearance Solid. (Crystalline solid.) Odor Not available.
Taste Not available.
Molecular Weight 158.05 g/mole
Color Not available.
pH (1% soln/water) Not available.
Boiling Point Not available.
Melting Point 116°C (240.8°F)
Critical Temperature Not available.
Not available.
Specific Gravity
Vapor Pressure Not applicable.
Vapor Density Not available.
Volatility Not available.
Odor Threshold Not available.
Not available.
Water/Oil Dist. Coeff.
Ionicity (in Water) Not available.
Not available.
Dispersion Properties
Solubility Not available.
Section 10. Stability and Reactivity Data
Stability The product is stable.
Not available.
Instability Temperature
Conditions of Instability Excess heat, incompatible materials, dust generation
Incompatibility with various Reactive with oxidizing agents.
substances
Corrosivity Not available.
Urea Phosphate
Special Remarks on Not available.
Reactivity
Special Remarks on Not available.
Corrosivity
Polymerization Will not occur.
Section 11. Toxicological Information
Routes of Entry Inhalation. Ingestion.
Toxicity to Animals LD50: Not available.
LC50: Not available.
Chronic Effects on Humans Not available.
Other Toxic Effects on Slightly hazardous in case of skin contact (irritant), of ingestion, of inhalation.
Humans
Special Remarks on Not available.
Toxicity to Animals
Special Remarks on
Not available.
Chronic Effects on Humans
Special Remarks on other Acute Potential Health Effects:
Toxic Effects on Humans Skin: May cause skin irritation.
Eyes: Dust may cause eye irritation.
Inhalation: Dust may cause respiratory tract irritation.
Ingestion: Expected to be a low hazard for usual industrial handling. The toxicological properties of this
substance have not been fully investigated.
Section 12. Ecological Information
Ecotoxicity Not available.
BOD5 and COD Not available.
Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may
arise.
Toxicity of the Products The product itself and its products of degradation are not toxic.
of Biodegradation
Special Remarks on the Not available.
Products of Biodegradation
Section 13. Disposal Considerations
Waste Disposal Waste must be disposed of in accordance with federal, state and local environmental
control regulations.
Section 14. Transport Information
DOT Classification Not a DOT controlled material (United States).
Identification Not applicable.
Not applicable.
Special Provisions for
Transport
Urea Phosphate
DOT (Pictograms)
Section 15. Other Regulatory Information and Pictograms
No products were found.
Federal and State
Regulations
California California prop. 65: This product contains the following ingredients for which the State of California has found
to cause cancer which would require a warning under the statute: No products were found.
Proposition 65
Warnings
California prop. 65: This product contains the following ingredients for which the State of California has found
to cause birth defects which would require a warning under the statute: No products were found.
Other Regulations Not available.
WHMIS (Canada) Not controlled under WHMIS (Canada).
Other Classifications
DSCL (EEC) This product is not classified according S22- Do not breathe dust.
to the EU regulations. S24/25- Avoid contact with skin and eyes.
Health Hazard
HMIS (U.S.A.) 1 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
1 0 Reactivity
Health
Reactivity
0
Specific hazard
Personal Protection
E
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
TDG (Canada)
(Pictograms)
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves.
Lab coat.
Dust respirator. Be sure to use an
approved/certified respirator or
equivalent.
Urea Phosphate
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
这是一种无色透明的棱柱状晶体,易溶于水且其水溶液呈酸性;不溶于醚类、甲苯、四氯化碳和二恶烷中。
用途
它用作牛、羊、马反刍动物饲料添加物,还可作为阻燃剂、金属表面处理剂、清洗剂等。此外,它是优良的饲料添加剂,能够提供磷和非蛋白氮(尿素态氮)两种营养元素,特别适用于反刍动物饲用,能减缓牛、羊瘤胃和血液中氮的释放和传递速度,安全性比尿素高。它也是一种高浓度氮磷复合肥料,适用于碱性土壤,并对水稻、小麦、油菜等作物均有增产效果。除此之外,还可用作阻燃剂、金属表面处理剂、发酵营养剂、清洗剂和净化磷酸用助剂。
生产方法
二次结晶法:将含50%P2O5的湿法磷酸与60%(质量)的尿素溶液加入混合反应器中,在110℃条件下混合后,通过泵送入真空蒸发器中进行浓缩。随后离心分离并干燥,制得磷酸脲成品。为得到饲料级产品,可进行二次结晶。分离后的母液经浓缩后再返回混合反应器加以利用。化学方程式如下:
[ \text{H}_3\text{PO}_4 + \text{CO(NH}_2\text{)}_2 \rightarrow (\text{H}_3\text{PO}_4)\cdot(\text{CO(NH}_2\text{)}_2) ]
真空浓缩一步法:将含25%P2O5的稀磷酸(含6%杂质)用烧碱预处理并倾析以除去氟硅酸钠,然后将其加入有熔融尿素的间歇反应槽内,配料比为21g尿素对100g磷酸溶液。在此条件下,磷酸脲完全溶解,随后将稀溶液预热,再进入真空浓缩器(P<13.3×100 Pa),于60℃进行浓缩。然后送到结晶器中冷却至20℃析出结晶。料浆在母液澄清槽中倾析,产品经少量水洗涤、干燥后制得磷酸脲成品。同样地,化学方程式如下: [ \text{H}_3\text{PO}_4 + \text{CO(NH}_2\text{)}_2 \rightarrow (\text{H}_3\text{PO}_4)\cdot(\text{CO(NH}_2\text{)}_2) ]
反应信息
-
作为反应物:参考文献:名称:短氢键的温度相关氘四极耦合常数摘要:摘要 非常短的氢键普遍显示出氘核磁共振四极耦合常数与温度的大正相关性。我们提供了八个这样的系统的温度相关 NMR 数据,O⋯O 距离在 238 到 250 pm 之间,并表明我们可以通过密度泛函方法对温度相关性进行建模,只要适当注意分子间效应和模式间耦合。DOI:10.1016/j.molstruc.2005.10.053
-
作为产物:参考文献:名称:Cochet; Houdin, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1932, vol. 195, p. 326摘要:DOI:
-
作为试剂:参考文献:名称:Purification of phosphoric acid with urea and nitric acid摘要:我的发明涉及一种新的湿法磷酸净化方法,同时生产氮溶液。粗酸经已知方法用尿素处理,形成几乎不含粗酸中大部分杂质的尿素磷酸盐。尿素磷酸盐经浓硝酸处理,形成固体尿素硝酸盐和磷酸。纯化后的磷酸与尿素硝酸盐分离,作为产品取出。洗涤后的尿素硝酸盐用浓硝酸洗涤,然后与尿素磷酸盐反应。洗涤后的尿素硝酸盐然后用氨和水处理,形成氮溶液。公开号:US03967948A1
文献信息
-
磷酸脲的制备方法申请人:云南江磷集团股份有限公司公开号:CN104003909B公开(公告)日:2016-08-31
-
Effect of Milk Thistle on the Pharmacokinetics of Indinavir in Healthy Volunteers作者:Stephen C. Piscitelli、Elizabeth Formentini、Aaron H. Burstein、Raul Alfaro、Shyla Jagannatha、Judith FalloonDOI:10.1592/phco.22.8.551.33205日期:2002.5Study Objective. To characterize the pharmacokinetics of indinavir in the presence and absence of milk thistle and to determine the offset of any effect of milk thistle on indinavir disposition. Design. Prospective open-label drug interaction study. Setting. Outpatient clinic. Subjects. Ten healthy volunteers. Intervention. Blood samples were collected over 8 hours after the volunteers took four doses of indinavir 800 mg every 8 hours on an empty stomach for baseline pharmacokinetics. This dosing and sampling were repeated after the subjects took milk thistle 175 mg (confirmed to contain silymarin 153 mg, the active ingredient) 3 times/day for 3 weeks. After an 11-day washout, indinavir dosing and blood sampling were repeated to evaluate the offset of any potential interaction. Measurements and Main Results. Indinavir concentrations were measured by using a validated high-performance liquid chromatography method. The following pharmacokinetic parameters were determined: highest concentration (Cmax), hour-0 concentration, hour-8 concentration (C8), time to reach Cmax, and area under the plasma concentration-time curve over the 8-hour dosing interval (AUC8). Milk thistle did not alter significantly the overall exposure of indinavir, as evidenced by a 9% reduction in the indinavir AUC8 after 3 weeks of dosing with milk thistle, although the least squares mean trough level (C8) was significantly decreased by 25%. Conclusion. Milk thistle in commonly administered dosages should not interfere with indinavir therapy in patients infected with the human immunodeficiency virus.研究目的描述茚地那韦在奶蓟草存在和不存在的情况下的药代动力学特征,并确定奶蓟草对茚地那韦处置的任何影响的抵消作用。 设计。前瞻性开放标签药物相互作用研究。 环境。门诊。 受试者。十名健康志愿者。 干预。志愿者每8小时空腹服用四次茚地那韦800毫克后,在8小时内采集血液样本,以测定基线药代动力学。受试者连续3周每天3次服用奶蓟草175毫克(经证实含有水飞蓟素153毫克,活性成分)后,重复上述剂量和采样。经过11天的冲洗后,再次进行茚地那韦剂量和血液采样,以评估任何潜在相互作用的抵消情况。 测量和主要结果。茚地那韦浓度的测定采用经过验证的高效液相色谱法。测定了以下药代动力学参数:最高浓度(Cmax)、0小时浓度、8小时浓度(C8)、达到Cmax的时间以及8小时给药间隔的血浆浓度-时间曲线下面积(AUC8)。服用奶蓟草3周后,茚地那韦的AUC8降低了9%,但最小二乘法平均谷浓度(C8)显著降低了25%,这表明奶蓟草并未显著改变茚地那韦的总体暴露量。 结论在人类免疫缺陷病毒感染者中,常用剂量的奶蓟草不会干扰茚地那韦治疗。
-
Purification of phosphoric acid with oxalic acid申请人:Tennessee Valley Authority公开号:US04169882A1公开(公告)日:1979-10-02Impure wet-process phosphoric acid is treated with urea by known processes to produce relatively pure urea phosphate which is then treated with oxalic acid to give solid urea oxalate and purified phosphoric acid product. The urea oxalate is either treated with ammonia to give urea and ammonium oxalate or it is pyrolyzed to produce urea and oxamide.
-
Process for the manufacture of crystalline urea phosphate申请人:Chemicals & Phosphates, Ltd.公开号:US03936501A1公开(公告)日:1976-02-03The present invention relates to a process for the direct manufacture of crystalline urea phosphate. The process consists in the reaction of solid urea with ortho-phosphoric acid in a substantially anhydrous form. A concentrated phosphoric acid with above 90% by weight H.sub.3 PO.sub.4 can be used as starting reagent. A preheating of the phosphoric acid at 60.degree.-90.degree.C is preferred in order to induce the spontaneous reaction with the solid urea. The urea phosphate product obtained is ready for use without any further operation. Desired micronutrients such as Mg, Co, Fe, Zn, Cu, Mn may be incorporated in the initial ortho-phosphoric acid prior to the reaction with the solid urea.
-
Water-soluble fertilizers申请人:Triomf Fertilizers公开号:US04175943A1公开(公告)日:1979-11-27A water soluble mixed fertilizer composition in solid form and a method of producing the fertilizer composition. The fertilizer comprises a mixture of urea, phosphoric acid and at least one potassium salt selected from the class comprising potassium sulphate, potassium nitrate and potassium chloride. Ammonium salts selected from the class comprising ammonium nitrate and ammonium sulphate may optionally also be added to the mixtures. Solid fertilizer compositions possible according to this invention include compositions which contain between 5 and 42% nitrogen, between 2 and 15% phosphorus and between 1 and 38% potassium, these values being elemental mass as a percentage relative to the total mass of the composition.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
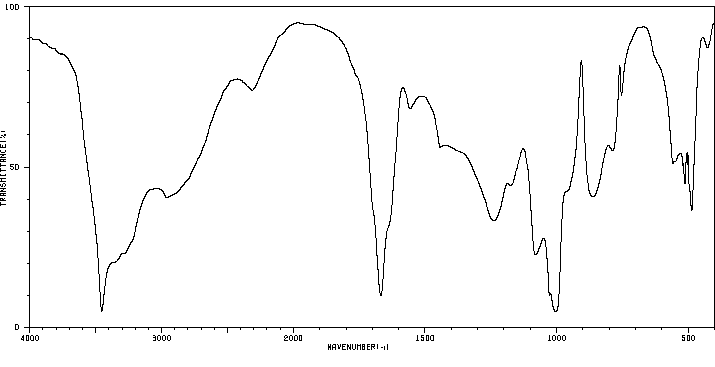
红外IR
-
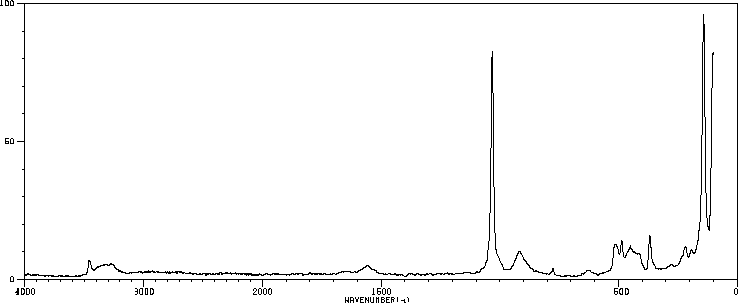
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息