2-氯-7-甲氧基-吩嗪 | 7293-97-2
中文名称
2-氯-7-甲氧基-吩嗪
中文别名
——
英文名称
2-chloro-7-methoxyphenazine
英文别名
2-chloro-7-methoxy-phenazine;2-Chlor-7-methoxy-phenazin;2-Chlor-6-methoxyphenazin
CAS
7293-97-2
化学式
C13H9ClN2O
mdl
——
分子量
244.68
InChiKey
DTEFZMLAXOFUQH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:173-174 °C(Solv: ethanol, 95% (64-17-5))
-
沸点:424.4±15.0 °C(Predicted)
-
密度:1.358±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:35
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:PHENAZINE SYNTHESES. II.1 PHENAZINE ETHERS AND PHENAZINOLS摘要:DOI:10.1021/jo01372a020
-
作为产物:描述:参考文献:名称:DIRECT RING CLOSURE THROUGH THE NITRO GROUP. ISOMER FORMATION IN THE SYNTHESIS OF UNSYMMETRICAL PHENAZINES, AND SOME GENERAL OBSERVATIONS ON THE PHENAZINE SYNTHESES摘要:DOI:10.1021/jo01141a001
文献信息
-
Reactivity and substituent effects in the cyclization of N -aryl-2-nitrosoanilines to phenazines作者:Zbigniew Wróbel、Karolina Plichta、Andrzej KwastDOI:10.1016/j.tet.2017.04.046日期:2017.6estimated on the base of the observed reaction times. A strong opposite effect of substituents located at position para to the nitroso group and those located para to the amino group in the side ring was observed. Mechanistic explanation, based on the electronic properties of the substituents and their mesomeric effects, was presented. The usefulness of the obtained data for the designed syntheses of
-
Notes - Phenazine Syntheses. VIII. Di-N-Oxides作者:Donald VivianDOI:10.1021/jo01115a607日期:1956.9
-
JPS5517341A申请人:——公开号:JPS5517341A公开(公告)日:1980-02-06
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
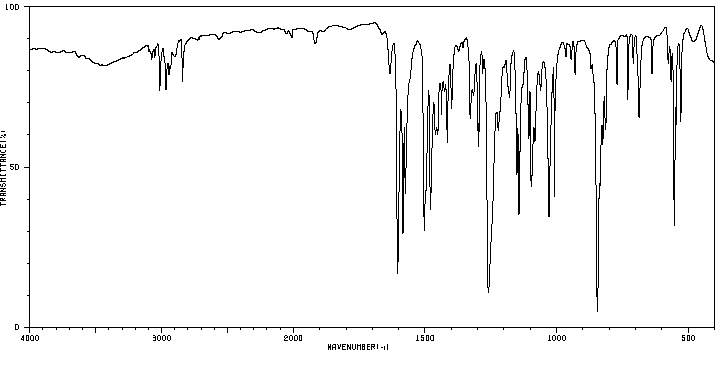
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮