N,N'-二甲基二硫代乙酰胺 | 120-79-6
中文名称
N,N'-二甲基二硫代乙酰胺
中文别名
N,N'-二甲基二硫草酰胺
英文名称
N,N'-dimethyldithiooxamide
英文别名
N,N'-Dimethyldithiooxamid;N,N'-dimethylethanedithioamide
CAS
120-79-6
化学式
C4H8N2S2
mdl
MFCD00022131
分子量
148.253
InChiKey
MQLIZYQZJMPNFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:88.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
海关编码:2930909090
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : N,N'-DIMETHYLDITHIOOXAMIDE
CAS-No. : 120-79-6
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Oral (Category 4)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful if swallowed.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H302 Harmful if swallowed.
Precautionary statement(s) none
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R22 Harmful if swallowed.
S-phrase(s) none
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C4H8N2S2
Molecular Weight : 148,25 g/mol
Component Concentration
N,N'-DIMETHYLDITHIOOXAMIDE
CAS-No. 120-79-6 -
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly
investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIRE-FIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx), Sulphur oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at
the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested and
approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the
standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges. Use
respirators and components tested and approved under appropriate government standards such as
NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 0,628
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation
May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly
investigated.
Additional Information
RTECS: RP0525000
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional
waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent
and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Walter,W.; Bode,K.-D., Justus Liebigs Annalen der Chemie, 1962, vol. 660, p. 74 - 84摘要:DOI:
-
作为产物:描述:参考文献:名称:Wallach, Justus Liebigs Annalen der Chemie, 1882, vol. 214, p. 259摘要:DOI:
文献信息
-
Bis(methylelement)dithiooxamid-derivate von Al, Ga, In, Si, Ge und Sn; planare metallabicyclen und verdrillte strukturen作者:T. Halder、W. Schwarz、J. Weidlein、P. FischerDOI:10.1016/s0022-328x(00)98648-0日期:1983.4When trimethyl derivatives of aluminium, gallium and indium are reacted with dithiooxamides, , in a molar ratio, 2 moles of methane are set free, and monomeric bis(dimethylmetal)/dithiooxamides are formed. By 1H NMR, IR and Raman spectroscopy, two structural isomers have been established for these metalla complexes, both with a planar molecular backbone of two fused five-membered rings, but different当铝,镓和铟的三甲基衍生物与二硫代草酰胺反应时,以摩尔比释放出2摩尔的甲烷,形成单体双(二甲基金属)/二硫代草酰胺。通过1 H NMR,IR和拉曼光谱,已经为这些金属配合物建立了两个结构异构体,两个异构体的平面分子骨架均为两个稠合的五元环,但两个金属原子的配位方式不同(E和Z型); 中心对称的E型也已经通过X射线晶体学表征。Si,Ge和Sn的等价双(三甲基元素)二硫代草酰胺是由三甲基氯化物与N,N'的反应获得的-二甲基二硫代乙酰胺。苯乙烯衍生物再次显示出平面双环结构原理。另一方面,对于硅和锗配合物,在晶体学上建立非螯合物结构,其中两个硫代羧酰胺部分旋转约80°。在溶液中,晶体变体异构化为三个旋转异构体的平衡混合物。根据1 H NMR光谱证据(例如,逐步信号合并)讨论了它们的构型。
-
Self‐Assembly of Hexameric Macrocycles from Pt <sup>II</sup> /Ferrocene Dimetallic Subunits – Synthesis, Characterization, Chemical Reactivity, and Oxidation Behavior作者:Antonino Giannetto、Fausto Puntoriero、Anna Notti、Melchiorre F. Parisi、Ileana Ielo、Francesco Nastasi、Giuseppe Bruno、Sebastiano Campagna、Santo LanzaDOI:10.1002/ejic.201501050日期:2015.12[(Me2SO)ClPt(HR2DTO κ‐S,S Pt)] (HR2DTO = secondary dithiooxamide, with R = methyl, ethyl, n‐propyl, n‐butyl, n‐decyl, isopropyl, (R)‐1‐phenylethyl, or (S)‐2‐hydroxypropyl; κ‐S,S Pt denotes the coordination of the DTO moiety to the Pt atom) have been prepared and used to synthesize platinum(II)/ferrocene dimetallic species IIa–h of formula [(dppf)Pt(HR2DTO κ‐S,S Pt)]Cl (dppf = 1,1′‐diphosphinoferrocene). Complexes IIa–e新的化合物IA - ħ形式的[(ME 2 SO)CLPT(HR 2 DTOκ-小号,小号的PT)](HR 2 DTO =仲二硫代草酰胺,其中R =甲基,乙基,Ñ丙基,Ñ丁基,ñ癸基,异丙基,(- [R)-1-苯乙基,或(小号)-2-羟基丙基;κ-小号,小号的PT表示DTO部分与铂原子的配)已经被制备并用于合成铂( II)/二茂铁二元金属物种IIA - ħ式[(DPPF)的PT(HR 2 DTOκ-小号,S PT)] Cl(DPPF = 1,1'-diphosphinoferrocene)。配合物IIa – e在二硫代乙酰胺部分带有直链基团(即R =甲基,乙基,正丙基,正丁基或正癸基),静置后自组装形成前所未有的六聚大环化合物IIIa – e。化合物IIa – e,IIIa – e和IIf – h的特征在于1 H,13 C,31的组合P NMR光谱以及2D-ROESY和扩散有序NMR光谱(D
-
Tight-Contact Ion Pairs Involving Pt(II) Dithiooxamide Complexes: the Acid−Base Reactions between Hydrohalogenated Ion-Paired Complexes and Pyridine作者:Antonino Giannetto、Fausto Puntoriero、Anna Barattucci、Santo Lanza、Sebastiano CampagnaDOI:10.1021/ic900875b日期:2009.11.2The equilibrium constants relative to HCl exchange between Pt(II)-containing tight contact ion pairs (TCIP) and pyridine have been investigated in chloroform solution at 298 K. The general formulas of the metal species are: [Pt(H2-R2-dithiooxamide)2]2+, 2Cl−} (a-type compounds; R = methyl (1a), ethyl (2a), n-propyl (3a), iso-propyl (4a), n-butyl (5a), cyclohexyl (6a), benzyl (7a), β-phenyl-ethyl (8a)已经在298 K的氯仿溶液中研究了含Pt(II)的紧密接触离子对(TCIP)和吡啶之间相对于HCl交换的平衡常数。金属物种的通式为:[Pt(H 2 -R 2-二硫代乙酰胺)2 ] 2 +,2Cl - }(a-型化合物; R =甲基(1a),乙基(2a),正丙基(3a),异丙基(4a),正丁基(5a) ,环己基(6a),苄基(7a),β-苯基乙基(8a),烯丙基(9a)))和[((HR 2 -dithiooxamidate)Pt(H 2 -R 2 -dithiooxamide)] +,Cl - }(b型化合物; R具有与以前相同的含义,产生1b - 9b种;此外,还研究了混合的R化合物10b,其在DTO(二硫代氨基甲酸酯/二硫代草酰胺)配体上含有R =苄基,在另一个DTO配体上含有R =乙基。此外,母体物种[Pt(HR 2 -dithiooxamidate)2 ](c型化合物;1c -
-
Effects of Uniaxial Pressure and Shear on the Electrical Conductivity of Solid. II. Electrical Conductivity in Some Amorphous Coordination Polymers作者:Seiichi Kanda、Kuwako Ohkawa、Kenichi Yamashita、Nobuo Oda、Hideaki ChiharaDOI:10.1246/bcsj.58.1619日期:1985.6Three amorphous coordination polymers, R2dtoaCu, where R=CH3, C6H5CH2, or cyclohexyl and dtoa=(N,N′-disubstituted dithiooxamidato), were prepared and the effect of uniaxial pressure on their electrical resistivities in the direction of the external pressure was measured. Application of the pressure up to 6×108 Pa caused an increase in the resistivity but, before reaching the equilibrium state, the resistivity exhibited a complicated time variation, which was not completely reversible upon pressure release as far as the measurement could trace. The results were explained on the basis of a model in which at least two competing processes occur. One of the processes is assumed to be reorientation of the two-dimensional network of molecules perpendicular to the applied pressure, which is a slow process and takes as long as hundreds of hours to reach the equilibrium. Activation energies of various processes were derived.## clewd修改版 v4.8(11) by tera **claude-3-5-sonnet-20241022 error**: 404 ```Not found``` FAQ: https://rentry.org/teralomaniac_clewd
-
Allylpalladium Dimers with Metals Connected by Binucleating Dithiooxamidates in Two Different Coordination Modes: Solution Behavior and Solid-State Structure作者:Santo Lanza、Francesco Nicolò、Hadi Amiri Rudbari、Maria Rosaria Plutino、Giuseppe BrunoDOI:10.1021/ic201616s日期:2011.11.21that follows the Pd–N bond rupture in the (η3-allyl)Pd(N^N) frame of kinetic compounds or in the (η3-allyl)Pd(N^S) frame of thermodynamic compounds. The dithiooxamidate [N(R)SC–CS(R)N]2–, when engaged in a κ-N,S Pd, κ-N′,S′ Pd′ coordination mode, behaves as a hybrid hemilabile binucleating ligand. At room temperature and in a chloroform solution, the kinetic compounds rearrange into the thermodynamically一系列烯丙基钯二聚体,其金属通过二烷基二硫代草酸二烷基酯[N(R)SC–CS(R)N] 2– [R =甲基,乙基,异丙基,苄基,异戊基,(S)-1-(1-苯基)连接而连接乙基,内消旋- (1-苯基)乙基,和外消旋- (1-苯基)乙基]是由monochelate [(η反应制备3 -烯丙基)钯(N(R)SC-CS(R)NHκ-S ,S的Pd)]与[(η 3 -烯丙基)的PdCl] 2在氯仿中。在低温(20℃)时,双金属配合物[(η 3 -烯丙基)的Pd] 2(μ-二烷基二硫代氨基甲酸酯κ-N,N'Pd,κ-S,S'Pd')(动力学化合物)在较短的反应时间(10分钟)内形成。在较高的温度(50℃)和较长的反应时间(24小时)时,相应的双金属的异构体[(η 3 -烯丙基)的Pd] 2获得(μ-二烷基二硫代草酰胺酸酯κ-N,S Pd,κ-N′,S′Pd′)(热力学化合物)。动力学和热力学化合物都可
表征谱图
-
氢谱1HNMR
-
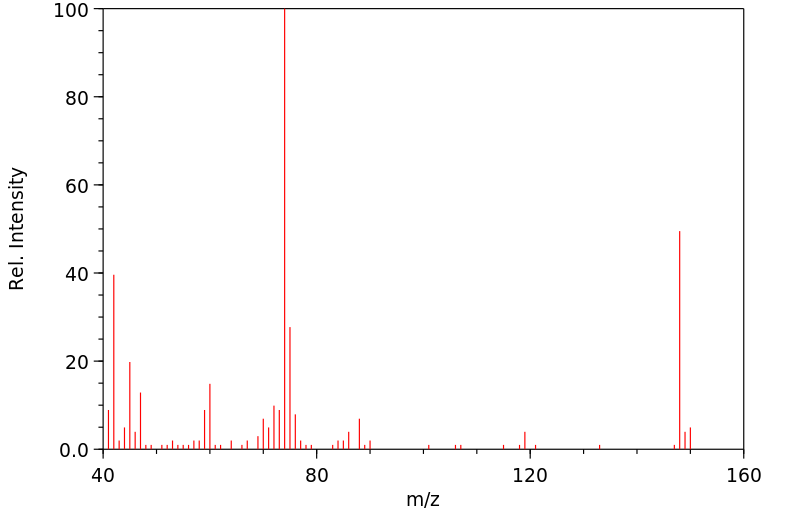
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
镉离子通道 I
铅离子载体III
硫脲与甲醛聚合物
硫代乙酰胺
硫代丙酰胺乙酯
硫代丙酰胺
环戊烷羟基硫胺
环丙烷硫代甲酰胺
环丁烷羟基硫胺
氰酸根硫杂酰胺,二-2-丙烯基-(9CI)
戊硫酸三甲基硅烷基甲基-酰胺
己硫代酰胺
双十二烷基二硫代乙二酰胺
二硫代乙酰胺
二甲胺基硫代乙酰胺盐酸盐
二异丙基二硫代氨基甲酸根
丙二硫代酰胺,2-乙基-
n-氰基-n-(2-甲基丙基)-硫脲
[2H9]-2,2-二甲基硫代丙酰胺
S-[5-(二甲基氨基)-5-硫代戊基]硫代乙酸酯
N-甲基乙烷二(硫代酰胺)
N-烯丙基-N,2-二甲基丙烷硫代酰胺
N-乙基硫代乙酰胺
N-(乙氧基羰基)硫代丙酰胺
N-(2-甲氧基乙基)-N-甲基硫代丙酰胺
N-(2-氨基-2-硫代乙基)乙酰胺
N,N-二甲基硫代乙酰胺
N,N-二甲基癸烷硫代酰胺
N,N-二甲基-10-十一碳烯硫代酰胺
N,N-二异丙基硫代丙酰胺
N,N-二异丙基乙烷硫代酰胺
N,N-二乙基丁烷硫代酰胺
N,N-二乙基-3-甲基硫代丁酰胺
N,N-二乙基-3-甲基-2-丁烯硫代酰胺
N,N-二乙基-2-甲基硫代丙酰胺
N,N-二乙基-2-(三甲基硅烷基)硫代乙酰胺
N,N-二乙基-2,2-二甲基丙烷硫代酰胺
N,N-二丙基-硫代丙酰胺
N,N-二丁基丁烷硫代酰胺
N,N,N',N'-四乙基二硫代草酰胺
N,N,N',N'-四(十二烷基)乙烷二硫代酰胺
N,N,3,3-四甲基硫代丁酰胺
N,N'-二甲基二硫代乙酰胺
N,N'-二环己基-二硫代乙酰胺
N,N'-二戊基乙烷二硫代酰胺
N,N'-二己基二硫代乙酰胺
N,N'-二丙基乙烷二硫代酰胺
N,N'-二[3-(二甲基氨基)丙基]二硫代草酰胺
N,N'-二[2-[乙基(3-甲基苯基)氨基]乙基]-1,2-二硫代乙烷-1,2-二胺
N,N'-二(辛基)乙烷二硫代酰胺