N,N-二甲基硫代乙酰胺 | 631-67-4
中文名称
N,N-二甲基硫代乙酰胺
中文别名
二甲基硫代乙酰胺
英文名称
N,N-dimethylthioacetamide
英文别名
N,N-dimethylethanethioamide
CAS
631-67-4
化学式
C4H9NS
mdl
MFCD00022178
分子量
103.188
InChiKey
LKNQXZAHNDFIQY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:71-73°C
-
沸点:125-127°C 22mm
-
密度:0.966
-
闪点:125-127°C/22mm
-
保留指数:1917
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:6
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:35.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险类别码:R36/37/38
-
海关编码:2930909090
-
储存条件:存放在密封容器中,并放置在阴凉、干燥处。请确保储存地点远离氧化剂。
SDS
Version 1.0
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name N,N-DIMETHYLTHIOACETAMIDE - 250 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Harmful if swallowed.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
N,N-DIMETHYLTHIOACETAMIDE 631-67-4 None None
Formula C4H9NS
Molecular Weight 103,1800 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
ALDRICH www.molbase.com
EXTINGUISHING MEDIA
Suitable: Carbon dioxide, dry chemical powder, or appropriate
foam. Water spray.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PERSONAL PRECAUTION PROCEDURES TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear self-contained breathing apparatus, rubber boots, and heavy
rubber gloves.
METHODS FOR CLEANING UP
Absorb on sand or vermiculite and place in closed containers for
disposal. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid prolonged or repeated
exposure. Do not breathe vapor. Avoid contact with eyes, skin,
and clothing.
STORAGE
Conditions of Storage: Keep tightly closed.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a full-face respirator with multi-purpose
combination (US) or type ABEK (EN 14387) respirator cartridges as
a backup to engineering controls. If the respirator is the sole
means of protection, use a full-face supplied air respirator.
Hand Protection: Compatible chemical-resistant gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Liquid
Property Value At Temperature or Pressure
ALDRICH www.molbase.com
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 0,398
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Sulfur oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
ACUTE TOXICITY
LD50
Intraperitoneal
Mouse
500 MG/KG
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: Material may be irritating to mucous membranes and
upper respiratory tract. May be harmful if inhaled.
Ingestion: Harmful if swallowed.
12 - Ecological Information
ALDRICH www.molbase.com
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
INDICATION OF DANGER: Xn
Harmful.
R-PHRASES: 22
Harmful if swallowed.
Caution: Substance not yet fully tested (EU).
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name N,N-DIMETHYLTHIOACETAMIDE - 250 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Harmful if swallowed.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
N,N-DIMETHYLTHIOACETAMIDE 631-67-4 None None
Formula C4H9NS
Molecular Weight 103,1800 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
ALDRICH www.molbase.com
EXTINGUISHING MEDIA
Suitable: Carbon dioxide, dry chemical powder, or appropriate
foam. Water spray.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PERSONAL PRECAUTION PROCEDURES TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear self-contained breathing apparatus, rubber boots, and heavy
rubber gloves.
METHODS FOR CLEANING UP
Absorb on sand or vermiculite and place in closed containers for
disposal. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid prolonged or repeated
exposure. Do not breathe vapor. Avoid contact with eyes, skin,
and clothing.
STORAGE
Conditions of Storage: Keep tightly closed.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a full-face respirator with multi-purpose
combination (US) or type ABEK (EN 14387) respirator cartridges as
a backup to engineering controls. If the respirator is the sole
means of protection, use a full-face supplied air respirator.
Hand Protection: Compatible chemical-resistant gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Liquid
Property Value At Temperature or Pressure
ALDRICH www.molbase.com
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 0,398
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide,
Sulfur oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
ACUTE TOXICITY
LD50
Intraperitoneal
Mouse
500 MG/KG
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: Material may be irritating to mucous membranes and
upper respiratory tract. May be harmful if inhaled.
Ingestion: Harmful if swallowed.
12 - Ecological Information
ALDRICH www.molbase.com
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
INDICATION OF DANGER: Xn
Harmful.
R-PHRASES: 22
Harmful if swallowed.
Caution: Substance not yet fully tested (EU).
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:使用Ph3SiSH(A至T)和Ph3SnOH(T至A)试剂进行酰胺(A)-硫酰胺(T)相互转化摘要:Ph 3 SiSH将酰胺转化为硫代酰胺,Ph 3 SnOH进行相反的过程,同时形成Ph 3 SiOH(或Ph 3 SiOSiPh 3)和Ph 3 SnSSnPh 3。化学性质很好地说明了硅的亲氧性与锡的亲硫性相比,并且发生在相对温和的条件下,对于酰胺到硫酰胺的转化,不需要酰胺活化。化学与用于硅可用数据一致(S)(O),锡(O)(S)和C(O)(S)键能。版权所有©2016 John Wiley&Sons,Ltd.DOI:10.1002/aoc.3462
-
作为产物:描述:N,N-二甲基乙酰胺 在 2,4-{[3-[(CH2)5C8F17]-4-MeO-phenyl]}2-P2S2 2,4-disulfide 作用下, 以 四氢呋喃 为溶剂, 反应 1.5h, 以73%的产率得到N,N-二甲基硫代乙酰胺参考文献:名称:氟代Lawesson试剂的合成和应用:方便的无色谱法产品纯化。摘要:[反应:见正文]已经开发了Lawesson试剂的羰基化合物硫磺化反应的氟类似物,并在一系列酰胺,酯和酮上证明了其用途。可以通过简单的氟固相萃取来分离Lawesson试剂衍生的副产物。DOI:10.1021/ol0529849
文献信息
-
A polymer-supported thionating reagent作者:Steven V. Ley、Andrew G. Leach、R. Ian StorerDOI:10.1039/b008814p日期:——A new polymer-supported reagent for the conversion of carbonyls to thiocarbonyls has been developed and its use demonstrated on a range of amides. Secondary or tertiary amides are converted cleanly and efficiently through to the corresponding thioamides and primary amides are converted to the corresponding nitriles. The reactions can be facilitated by conventional heating. However, if microwave heating
-
Studies on organophosphorus compounds XLVII preparation of thiated synthons of amides, lactams and imides by use of some new p,s-containing reagents作者:B. Yde、N.M. Yousif、U. Pedersen、I. Thomsen、S.-O. LawessonDOI:10.1016/s0040-4020(01)88445-3日期:1984.14-bis(4-phen-oxyphenyl)-1,3,2,4-dithiaphosphetan is that 2, 3 and thionate most amides and lactams In THF at room temperature (reaction time 5 min) to give the corresponding thionated compounds. Imides are easily thionated by 2, 3 and 4 In DME at 60 °C. The reactions of 1 with amides, imides and most lactams are run at 60°C to give good yields of the corresponding thionated compounds.
-
Coupling reaction of thioamides with sulfonyl azides: an efficient catalyst-free click-type ligation under mild conditions作者:Muhammad Aswad、Junya Chiba、Takenori Tomohiro、Yasumaru HatanakaDOI:10.1039/c3cc46055j日期:——We report a coupling reaction of thioamides and sulfonyl azides to generate sulfonyl amidines in the absence of any activation additives. The reaction progresses in various solvents under mild conditions. Water exhibits the highest performance with respect to efficiency.
-
Synthesis of Tertiary Propargylamines by Sequential Reactions of in Situ Generated Thioiminium Salts with Organolithium and -magnesium Reagents作者:Toshiaki Murai、Yuichiro Mutoh、Yukiyasu Ohta、Masahiro MurakamiDOI:10.1021/ja048627v日期:2004.5.1Sequential reactions of thioiminium salts generated from thioamides and methyl triflate with organolithium and -magnesium reagents proceed smoothly to give tertiary propargylamines in moderate to high yields. In all cases, two different organometallic reagents are incorporated into the starting thioamides.
-
Nickel-catalyzed <i>C</i>-alkylation of thioamide, amides and esters by primary alcohols through a hydrogen autotransfer strategy作者:Peng Yang、Xiuhua Wang、Yu Ma、Yaxin Sun、Li Zhang、Jieyu Yue、Kaiyue Fu、Jianrong Steve Zhou、Bo TangDOI:10.1039/d0cc06468h日期:——A simple catalyst of Ni(OAc)2 and P(t-Bu)3 enables selective C-alkylation of thioacetamides and primary acetamide with alcohols for the first time. Monoalkylation of thioamides, amides and t-butyl esters occurs in excellent yields (>95%). Mechanistic studies reveal that the reaction proceeds via a hydrogen autotransfer pathway.
表征谱图
-
氢谱1HNMR
-
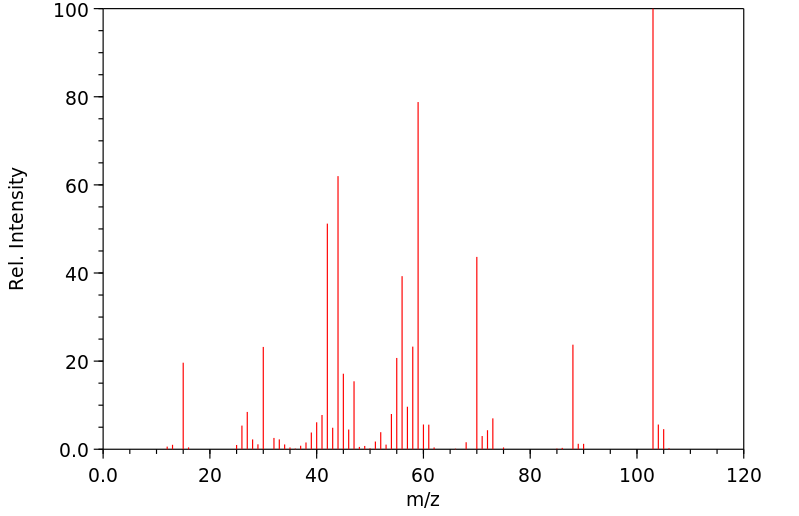
质谱MS
-
碳谱13CNMR
-
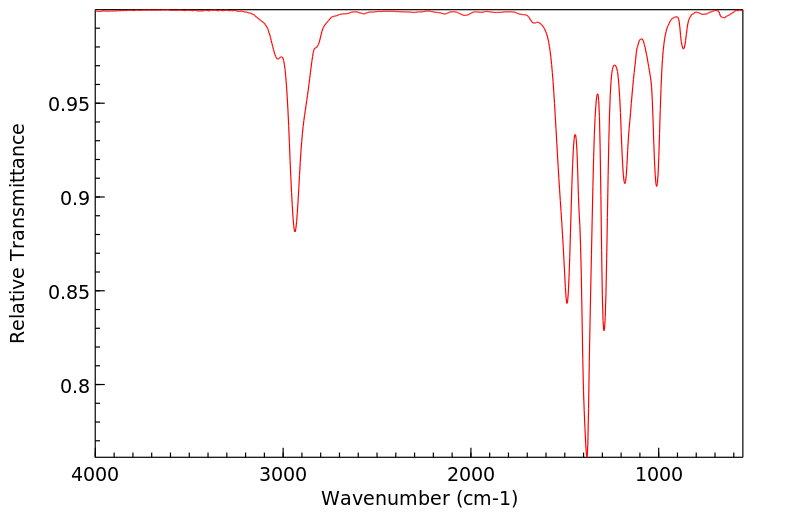
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
镉离子通道 I
铅离子载体III
硫脲与甲醛聚合物
硫代乙酰胺
硫代丙酰胺乙酯
硫代丙酰胺
环戊烷羟基硫胺
环丙烷硫代甲酰胺
环丁烷羟基硫胺
氰酸根硫杂酰胺,二-2-丙烯基-(9CI)
戊硫酸三甲基硅烷基甲基-酰胺
己硫代酰胺
双十二烷基二硫代乙二酰胺
二硫代乙酰胺
二甲胺基硫代乙酰胺盐酸盐
二异丙基二硫代氨基甲酸根
丙二硫代酰胺,2-乙基-
n-氰基-n-(2-甲基丙基)-硫脲
[2H9]-2,2-二甲基硫代丙酰胺
S-[5-(二甲基氨基)-5-硫代戊基]硫代乙酸酯
N-甲基乙烷二(硫代酰胺)
N-烯丙基-N,2-二甲基丙烷硫代酰胺
N-乙基硫代乙酰胺
N-(乙氧基羰基)硫代丙酰胺
N-(2-甲氧基乙基)-N-甲基硫代丙酰胺
N-(2-氨基-2-硫代乙基)乙酰胺
N,N-二甲基硫代乙酰胺
N,N-二甲基癸烷硫代酰胺
N,N-二甲基-10-十一碳烯硫代酰胺
N,N-二异丙基硫代丙酰胺
N,N-二异丙基乙烷硫代酰胺
N,N-二乙基丁烷硫代酰胺
N,N-二乙基-3-甲基硫代丁酰胺
N,N-二乙基-3-甲基-2-丁烯硫代酰胺
N,N-二乙基-2-甲基硫代丙酰胺
N,N-二乙基-2-(三甲基硅烷基)硫代乙酰胺
N,N-二乙基-2,2-二甲基丙烷硫代酰胺
N,N-二丙基-硫代丙酰胺
N,N-二丁基丁烷硫代酰胺
N,N,N',N'-四乙基二硫代草酰胺
N,N,N',N'-四(十二烷基)乙烷二硫代酰胺
N,N,3,3-四甲基硫代丁酰胺
N,N'-二甲基二硫代乙酰胺
N,N'-二环己基-二硫代乙酰胺
N,N'-二戊基乙烷二硫代酰胺
N,N'-二己基二硫代乙酰胺
N,N'-二丙基乙烷二硫代酰胺
N,N'-二[3-(二甲基氨基)丙基]二硫代草酰胺
N,N'-二[2-[乙基(3-甲基苯基)氨基]乙基]-1,2-二硫代乙烷-1,2-二胺
N,N'-二(辛基)乙烷二硫代酰胺