bis(naphthalen-2-yl) carbonate | 89784-86-1
中文名称
——
中文别名
——
英文名称
bis(naphthalen-2-yl) carbonate
英文别名
di(naphthalen-2-yl) carbonate;carbonic acid di-[2]naphthyl ester;Kohlensaeure-di-[2]naphthylester;Di-(naphthyl-(2))-carbonat;Kohlensaeure-di-β-naphthylester;Di-β-naphthyl-carbonat;Carbonic acid, di(2-naphthyl) ester;dinaphthalen-2-yl carbonate
CAS
89784-86-1
化学式
C21H14O3
mdl
——
分子量
314.34
InChiKey
VJJMAHPIEWRZKY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:175 °C
-
沸点:490.3±14.0 °C(Predicted)
-
密度:1.271±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:24
-
可旋转键数:4
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 氯甲酸-2-萘酯 2-naphthyl chloroformate 7693-50-7 C11H7ClO2 206.628
反应信息
-
作为反应物:描述:bis(naphthalen-2-yl) carbonate 在 alkali carbonate 作用下, 生成 14H-二苯并[a,j]氧杂蒽-14-酮参考文献:名称:Fosse, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1904, vol. 138, p. 1053摘要:DOI:
-
作为产物:描述:参考文献:名称:Manufacturie of aromatic esters摘要:公开号:US02362865A1
文献信息
-
Oxidative carbonylation of monohydroxy aryl compounds by methyl formate申请人:Bayer MaterialScience AG公开号:EP2781503A1公开(公告)日:2014-09-24The field of the present invention relates to a process for continuously preparing alkylaryl carbonates and diarylcarbonates from methyl formate and at least one monohydroxy aryl compound in the presence of catalysts, and to the use thereof for preparation of polycarbonates.
-
Photo-on-Demand Base-Catalyzed Phosgenation Reactions with Chloroform: Synthesis of Arylcarbonate and Halocarbonate Esters作者:Yuka Hashimoto、Sasuga Hosokawa、Fengying Liang、Yuto Suzuki、Namin Dai、Gegen Tana、Kazuo Eda、Toshifumi Kakiuchi、Takashi Okazoe、Hidefumi Harada、Akihiko TsudaDOI:10.1021/acs.joc.1c01210日期:2021.7.16utilized as solvents and reagents for C1 building blocks in organic synthesis. This study reports a novel photo-on-demand in situ synthesis of carbonate esters with CHCl3 solutions containing a mixture of an aromatic or haloalkyl alcohol having relatively high acidity, and an organic base. We found that the acid–base interaction of the alcohol and base in the CHCl3 solution plays a key role in enabling the
-
Synthesis of Polyalkylphenyl Prop-2-ynoates and Their Flash Vacuum Pyrolysis to Polyalkylcyclohepta[b]furan-2(2H)-ones作者:Matthias Nagel、Hans-Jürgen HansenDOI:10.1002/(sici)1522-2675(20000510)83:5<1022::aid-hlca1022>3.0.co;2-y日期:2000.5.10A new method for the smooth and highly efficient preparation of polyalkylated aryl propiolates has been developed. It is based on the formation of the corresponding aryl carbonochloridates (cf. Scheme 1 and Table 1) that react with sodium (or lithium) propiolate in THF at 25 – 65°, with intermediate generation of the mixed anhydrides of the arylcarbonic acids and prop-2-ynoic acid, which then decompose开发了一种平稳高效制备多烷基化芳基丙酸酯的新方法。它基于形成相应的芳基碳酰氯(参见方案 1 和表 1),其与 THF 中 25-65° 的丙醇钠(或锂)反应,中间生成芳基碳酸和丙醇的混合酸酐-2-ynoic acid,然后几乎定量地分解成 CO2 和芳基丙酸酯(参见方案 11)。该过程优于将丙炔酸转化为其难以处理的酰氯,然后与芳醇钠(或锂)反应。许多多烷基化芳基丙炔酸酯在 600 – 650° 和 10-2 Torr 下进行闪蒸真空热解 (FVP),导致形成相应的环庚[b]furan-2(2H)-ones,平均产率为25 – 45%(参见方案 14)。在中试实验中进一步发现,多烷基化的 cyclohepta[b]furan-2(2H)-ones 与甲苯中的 1-(pyrrolidin-1-yl)cyclohexene 在 120 – 130° 下反应生成相应的 1,2, 3,4-四氢苯并[
-
PROCESSES FOR PRODUCING ARYL CARBAMATES, ISOCYNATES AND POLYUREAS USING DIARYL CARBONATE申请人:Dai Shenghong A.公开号:US20130079542A1公开(公告)日:2013-03-28A preparation of aryl carbamates can be achieved readily by carbonylation of an aromatic polyamine compound with diphenyl carbonate (DPC) using a combination of an organic acid and a tertiary amine as a catalyst. Aryl carbamate can be converted into 4,4′-diphenylmethane diisocyanate (MDI) by heating it at about 200 to about 230° C. in a non-polar solvent containing inhibitor such as benzoyl chloride. In another application, trans-ureation of biscarbamates with an amine or mixed amines is found to be extremely facile in a polar solvent such as dimethyl sulfoxide (DMSO) and tetramethylene sulfone (TMS) in absence of any catalyst to make polyurea polymers of high molecular weights. Thus, efficient green-chemistry processes based on biscarbamates in making isocyanate products as well as urea prepolymers, urea elastomers and urea plastics have been developed in all in excellent yields without using reactive phosgene or 4,4′-diphenylmethane diisocyanate separately in the trans-ureation polymerizations.芳基氨基甲酸酯的制备可通过芳香族多胺化合物与二苯基碳酸酯(DPC)在有机酸和三级胺的催化剂作用下进行羰基化而轻松实现。将芳基氨基甲酸酯加热至约200至230°C,在含有苯甲酰氯等抑制剂的非极性溶剂中,可将其转化为4,4′-二苯甲烷二异氰酸酯(MDI)。在另一个应用中,使用极性溶剂如二甲基亚砜(DMSO)和四甲基砜(TMS)中,通过与胺或混合胺进行双氨基甲酸酯的转尿素化,在无催化剂的情况下制备高分子量的聚脲聚合物是非常容易的。因此,基于双氨基甲酸酯的高效绿色化学过程已经开发出以优异产率制备异氰酸酯产品、尿素预聚合物、尿素弹性体和尿素塑料的方法,而无需在转尿素聚合过程中单独使用反应性的光气或4,4′-二苯甲烷二异氰酸酯。
-
PROCESS FOR PREPARING DIARYL CARBONATES FROM DIALKYL CARBONATES申请人:Düx Andre公开号:US20100010252A1公开(公告)日:2010-01-14The invention provides a process for preparing diaryl carbonates from dialkyl carbonates and aromatic hydroxyl compounds using at least two reaction columns, a process section for recovering the dialkyl carbonate used in the reaction and for removing the alcohol of reaction, one or more process steps for removing the by-products obtained in the process which have a boiling point between that of the dialkyl carbonate and that of the alkyl aryl carbonate formed during the preparation of the diaryl carbonate, and a process step for further purification of the diaryl carbonate obtained from the reaction columns.
表征谱图
-
氢谱1HNMR
-
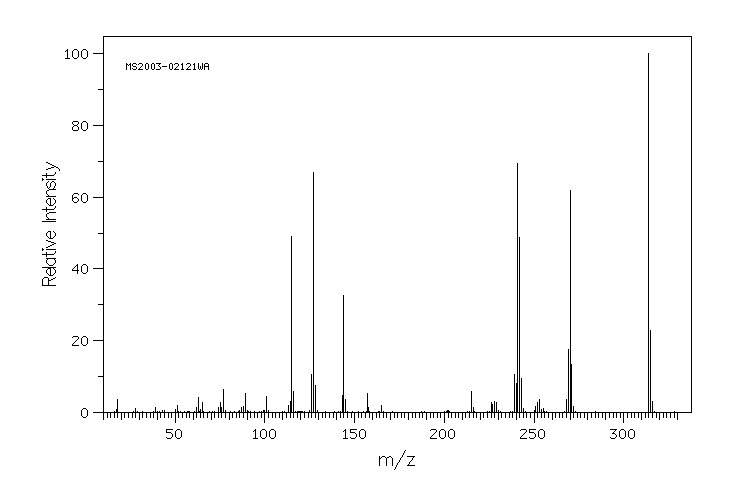
质谱MS
-
碳谱13CNMR
-
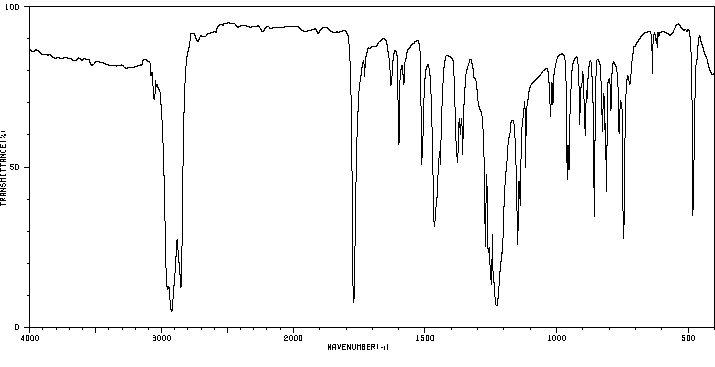
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮