邻亚苯基二苯甲酸酯 | 643-94-7
分子结构分类
中文名称
邻亚苯基二苯甲酸酯
中文别名
——
英文名称
o-phenylene dibenzoate
英文别名
catechol dibenzoate;Pyrocatechol-dibenzoat;(2-benzoyloxyphenyl) benzoate
CAS
643-94-7
化学式
C20H14O4
mdl
MFCD00128083
分子量
318.329
InChiKey
LVTPRIAGCBEGPW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:24
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2916399090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Lipase Ps-C Catalysed Hydrolysis Of Aryl Deesters: A New Route To The Synthesis Of Achiral Half Esters摘要:Pseudomonas cepacia lipase supported on ceramic particles (PS-C) offers a simple alternative route for the synthesis of achiral half esters, with very high yields, easy work up and remarkable substrate selectivity, as it cleaves only phenolic esters having a phenyl group (i.e. C6H5-O-CO-C6H5).DOI:10.1080/00397919908086153
-
作为产物:描述:2-溴-2-苯基乙酰苯 在 吡啶 、 氯化亚砜 、 氧气 、 sodium ethanolate 、 rose bengal 作用下, 以 二氯甲烷 、 xylene 为溶剂, 反应 9.0h, 生成 邻亚苯基二苯甲酸酯参考文献:名称:Synthesis, thermal stability, and chemiluminescence properties of the dioxetanes derived from 1,4-dioxins摘要:DOI:10.1021/jo00195a008
文献信息
-
HF–Pyridine: A Versatile Promoter for Monoacylation/Sulfonylation of Phenolic Diols and for Direct Conversion of <i>t</i>-Butyldimethylsilyl Ethers to the Corresponding Acetates作者:Kyosuke Michigami、Kazuya Yoshimoto、Masahiko HayashiDOI:10.1246/cl.2012.138日期:2012.2.5Monoacylation and trifluoromethanesulfonylation of phenolic diols were achieved by the aid of HF–pyridine, whereas diacylation occurred with pyridine alone. Furthermore, HF–pyridine was found to promote the direct conversion of t-butyldimethylsilyl ethers to the corresponding acetates.
-
Scandium Trifluoromethanesulfonate as an Extremely Active Lewis Acid Catalyst in Acylation of Alcohols with Acid Anhydrides and Mixed Anhydrides作者:Kazuaki Ishihara、Manabu Kubota、Hideki Kurihara、Hisashi YamamotoDOI:10.1021/jo952237x日期:1996.1.1is commercially available, is a practical and useful Lewis acid catalyst for acylation of alcohols with acid anhydrides or the esterification of alcohols by carboxylic acids in the presence of p-nitrobenzoic anhydrides. The remarkably high catalytic activity of scandium triflate can be used for assisting the acylation by acid anhydrides of not only primary alcohols but also sterically-hindered secondary
-
Highly Powerful and Practical Acylation of Alcohols with Acid Anhydride Catalyzed by Bi(OTf)<sub>3</sub>作者:Akihiro Orita、Chiaki Tanahashi、Atsushi Kakuda、Junzo OteraDOI:10.1021/jo0107453日期:2001.12.1Bi(OTf)(3)-catalyzed acylation of alcohols with acid anhydride was evaluated in comparison with other acylation methods. The Bi(OTf)(3)/acid anhydride protocol was so powerful that sterically demanding or tertiary alcohols could be acylated smoothly. Less reactive acylation reagents such as benzoic and pivalic anhydride are also activated by this catalysis. In these cases, a new technology was developed
-
Electrostatic catalysis by ionic aggregates: scope and limitations of Mg(ClO4)2 as acylation catalyst作者:Asit K Chakraborti、Lalima Sharma、Rajesh Gulhane、ShivaniDOI:10.1016/j.tet.2003.08.007日期:2003.9Alkali and alkaline earth metal perchlorates exhibit electrostatic catalysis in the activation of anhydrides for the acylation reaction. Perchlorates with higher values of the charge-size function of the metal ion exhibit better catalytic activity following the order Mg(ClO4)2>Ba(ClO4)2>LiClO4. Acylation of structurally diverse phenols, thiols, alcohols, and amines have been carried out with stoichiometric
-
Molybdenum Hexacarbonyl Mediated Alkoxycarbonylation of Aryl Halides作者:Motoki Yamane、Wei Ren、A. EmiDOI:10.1055/s-0030-1260060日期:2011.7Mo(CO)6-mediates the alkoxycarbonylation of aryl halides in their reaction with alcohols to afford arenecarboxylic acid esters. The molybdenum carbonyl complexes act as the catalyst and the source with carbon monoxide. The alkoxycarbonylation proceeds with a small excess of carbon monoxide in the form of Mo(CO)6 and the procedure is simple compared to the conventional method, which uses palladium catalyst
表征谱图
-
氢谱1HNMR
-
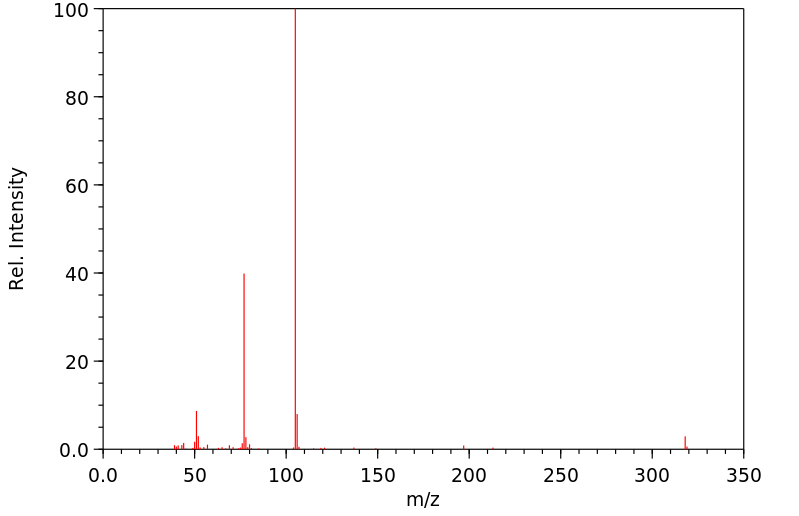
质谱MS
-
碳谱13CNMR
-
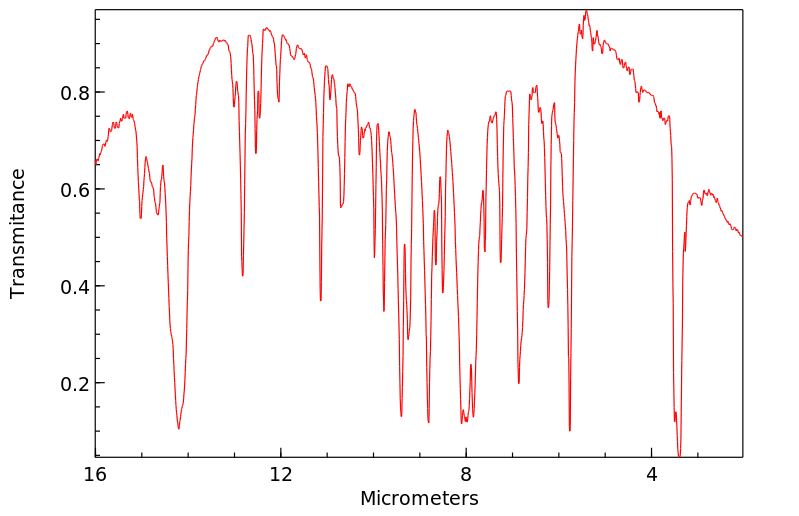
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
非那米柳
雷尼替丁
降钙素(humanreduced),8-L-缬氨酸-(9CI)
间苯甲酰氧基苯乙酮
间苯二甲酸二苯酯
间甲苯基苯甲酸酯
间双没食子酸
醋氨沙洛
邻苯二甲酸苄酯2-乙己基酯
邻苯二甲酸二苯酯-D4
邻苯二甲酸二苯酯
邻甲苯基苯甲酸酯
邻氨基苯甲酸(4-硝基苯基)酯
邻亚苯基二苯甲酸酯
贝诺酯
袋衣酸
血竭黄烷A
萘-1,5-二磺酸-4-[2-(二甲氨基)乙氧基]-2-甲基-5-(丙烷-2-基)苯基2-氨基苯酸酯(1:1)
茶痂衣酸
苯酚,2-[2-[(4-氯苯基)氨基]-4-噻唑基]-,苯酸酯(ester)
苯甲醯柳酸甲酯
苯甲酸苯酯
苯甲酸五氟苯酯
苯甲酸丁香酚酯
苯甲酸4-[[(4-甲氧基苯基)亚甲基]氨基]苯基酯
苯甲酸4-(乙酰氨基)-2-[[2-[4-(乙酰氨基)苯甲酰基]亚肼基]甲基]苯基酯
苯甲酸2-(2-苯并恶唑基)苯酯
苯甲酸-4-甲基苯酯
苯甲酸-(4-环戊基-苯基酯)
苯甲酸-(2-烯丙基-4-溴-苯基酯)
苯甲酸-(2-溴-4,6-二硝基-苯基酯)
苯甲酸-(2,4-二溴-3-甲基-苯基酯)
苯甲酸-(2,4-二氯-5-甲基-苯基酯)
苯甲酸-(2,4-二叔丁基苯基酯)
苯甲酸,4-羟基-,4-[(4-羟基苯氧基)羰基]苯基酯
苯甲酸,4-羟基-,4-(己氧基)苯基酯
苯甲酸,4-羟基-,4-(十四烷氧基)苯基酯
苯甲酸,4-羟基-,4-(十二烷氧基)苯基酯
苯甲酸,4-甲酰基-,4-(辛氧基)苯基酯
苯甲酸,4-甲氧基-,2-甲酰基苯基酯
苯甲酸,4-甲基-,4-甲基苯基酯
苯甲酸,4-戊基-,4-(壬氧基)苯基酯
苯甲酸,4-丁氧基-,1,4-亚苯基酯
苯甲酸,4-[[[3-[(2,2-二甲基-1-羰基丙氧基)甲基]-3,4-二氢-2-甲基-4-羰基-6-喹唑啉基]甲基]-2-炔丙基氨基]-,五氟苯基酯
苯甲酸,4-[1-(己氧基)乙基]-,4-(辛氧基)苯基酯
苯甲酸,4-(辛氧基)-,4-[[4-[[(1-甲基庚基)氧代]羰基]苯基]乙炔基]苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(癸氧基)苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(壬氧基)苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(十二烷氧基)苯基酯
苯甲酸,4-(癸氧基)-,4-[氰基[(1-羰基戊基)氧代]甲基]苯基酯,(R)-