triphenyl isocyanurate | 1785-02-0
中文名称
——
中文别名
——
英文名称
triphenyl isocyanurate
英文别名
1,3,5-triphenyl-1,3,5-triazinane-2,4,6-trione;1,3,5-triphenylisocyanurate;1,3,5-triphenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione;1,3,5-triphenylperhydro-1,3,5-triazine-2,4,6-trione
CAS
1785-02-0
化学式
C21H15N3O3
mdl
——
分子量
357.368
InChiKey
YEACGXMAEGBJSM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:275 °C
-
沸点:490°C (rough estimate)
-
密度:1.3023 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:27
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:60.9
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933699090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3,5-tris-(4-nitrophenyl)-[1,3,5]triazine-2,4,6-trione 20607-39-0 C21H12N6O9 492.361
反应信息
-
作为反应物:参考文献:名称:Stolle, Chemische Berichte, 1908, vol. 41, p. 1126摘要:DOI:
-
作为产物:描述:异氰酸苯酯 在 1-(3-phenylpropyl)-3-pentylbenzimidazolium bromide 作用下, 以 neat (no solvent) 为溶剂, 反应 0.08h, 以99%的产率得到triphenyl isocyanurate参考文献:名称:苯并咪唑盐原位制备的N-杂环卡宾在Suzuki-Miyaura交叉偶联反应中的催化作用及其在1,3,5-三苯基-1,3,5-三嗪2,4,6-的催化制备中的应用异氰酸苯酯中的三酮摘要:合成了许多带有3-苯基丙基取代基(1a-1h)的苯并咪唑盐,并通过1 H NMR,13 C NMR,IR光谱法和元素分析鉴定了它们的结构。这些N-杂环卡宾(NHC)前体在微波辐射下被用作Sduki-Miyaura交叉偶联反应中包括Pd(OAc)2和碱的催化体系的一部分。它们还用作异氰酸苯酯的环三聚反应的催化剂,得到1,3,5-三苯基-1,3,5-三嗪烷-2,4,6-三酮。已经观察到,苯并咪唑盐作为NHC前体对两种催化反应均具有积极作用。DOI:10.3906/kim-1804-39
文献信息
-
1,4,2-Diazaphospholidine-3,5-diones and Related Compounds: A Lecture on Unpredictability in Catalysis作者:Frank U. RichterDOI:10.1002/chem.200900039日期:2009.5.18Whereas 1‐organyl‐phospholanes and 1‐organyl‐2,3‐dihydro‐1H‐phospholes catalyze isocyanate oligomerization, the reaction of isocyanates with 1‐organyl‐2,5‐dihydro‐1H‐phospholes results in the formation of 1,3‐dienes and a novel class of P‐heterocycles, 1,4,2‐diazaphospholidine‐3,5‐diones. Isothiocyanates and carbodiimides exhibit analogous behavior. The resultant species readily form P‐oxides, P‐sulfides
-
Synergistic Catalysis by Brønsted Acid/Carbodicarbene Mimicking Frustrated Lewis Pair‐Like Reactivity作者:Yi‐Chen Chan、Yuna Bai、Wen‐Ching Chen、Hsing‐Yin Chen、Chen‐Yu Li、Ying‐Yann Wu、Mei‐Chun Tseng、Glenn P. A. Yap、Lili Zhao、Hsuan‐Ying Chen、Tiow‐Gan OngDOI:10.1002/anie.202107127日期:2021.9Carbodicarbene (CDC), unique carbenic entities bearing two lone pairs of electrons are well-known for their strong Lewis basicity. We demonstrate herein, upon introducing a weak Brønsted acid benzyl alcohol (BnOH) as a co-modulator, CDC is remolded into a Frustrated Lewis Pair (FLP)-like reactivity. DFT calculation and experimental evidence show BnOH loosely interacting with the binding pocket of CDC碳二碳烯 (CDC) 是一种独特的碳烯实体,带有两对孤对电子,以其强路易斯碱度而闻名。我们在此证明,在引入弱 Brønsted 酸苄醇 (BnOH) 作为共调节剂后,CDC 被重塑为类似受挫路易斯对 (FLP) 的反应性。DFT 计算和实验证据表明,BnOH 通过 H 键合和 π-π 堆积与 CDC 的结合口袋松散地相互作用。部署了四种不同的自然界反应,以证明作为协同 FLP/调节剂 (CDC/BnOH) 的概念验证的可行性,证明异氰酸酯环三聚、L聚合过程中的催化反应性增强-丙交酯 (LA)、甲基丙烯酸甲酯 (MMA) 和醇的脱氢硅烷化。重要的是,碳二碳烯的催化反应性与仅依赖于亲核性的单一化学特征的常规 NHC 截然不同。这一发现还为通过共调节剂或助催化剂使 FLP 反应性多样化提供了新的思路。
-
Chloroboration and allied reactions of unsaturated compounds. Part VIII. Insertion of isocyanates and isothiocyanates into Al–Et and Al–Br bonds作者:J. R. Horder、M. F. LappertDOI:10.1039/j19680002004日期:——co-ordination compounds (formulated as RNC[graphic omitted]–l[graphic omitted]Br3, and characterised by an abnormally-high NCO asymmetric stretching frequency at ca. 2400 cm.–1) were identified as the first-formed products. The insertion compounds were dimeric in solution and are assigned an eight-membered ring structure. Exceptions are Br2AlN(Ph)C(:Q)Br (Q = O or S), which are monomeric, and are believed考察了三乙基铝,氯化乙基铝(Et-Al裂解)和三溴化铝与异氰酸酯和异硫氰酸酯(主要是PhNCO和PhNCS)的反应。在温和的条件下,在每种情况下均会分离出固体1:1插入(插入到Et-Al或Br-Al键中)[例如,从Al 2 Br 6和2PhNCO中的Br 2 AlN(Ph)C(:O)Br ]。用Al 2溴6和任一PhNCO或MeNCO亚稳协调化合物(如RNC [图形省略]配制-l [图形省略]溴3,并且其特征在于异常高NCO不对称在伸缩频率约2400厘米。- 1个)被确定为最早形成的产品。插入化合物在溶液中为二聚体,并被分配为八元环结构。Br 2 AlN(Ph)C(:Q)Br(Q = O或S)是例外,它们是单体的,被认为是螯合物,具有四坐标铝。
-
The introduction of nitrile-groups into heterocycles and conversion of carboxylic groups into their corresponding nitriles with chlorosulfonylisocyanate and triethylamine作者:Helmut Vorbrüggen、Konrad KrolikiewiczDOI:10.1016/s0040-4020(01)89685-x日期:——Addition of chlorosulfonylisocyanate (CSI) to heterocycles such as thiophene (4) or indole (15) and unsaturated systems such as dihydropyran (7) gives N-chlorosulfonylamides RCONHSO2Cl, which can be converted by equivalent amounts of triethylamine to their corresponding nitriles. Since carboxylic acids react with CSI to N-chlorosulfonylamides, subsequent treatment with triethylamine affords the corresponding
-
The Synthesis of Isocyanurates on the Trimerization of Isocyanates under High Pressure作者:Yoichi Taguchi、Isao Shibuya、Masahiko Yasumoto、Tohru Tsuchiya、Katsumi YonemotoDOI:10.1246/bcsj.63.3486日期:1990.12The trimerization of phenyl isocyanate in the presence of triethylamine was accelerated under high pressure to give triphenyl isocyanurate almost quantitatively. The reaction in benzene was remarkably accelerated by compression. The effects of pressure, temperature, catalysts, and solvents were examined on the trimerization of phenyl isocyanate. Aryl and normal alkyl isocyanates trimerized under high
表征谱图
-
氢谱1HNMR
-
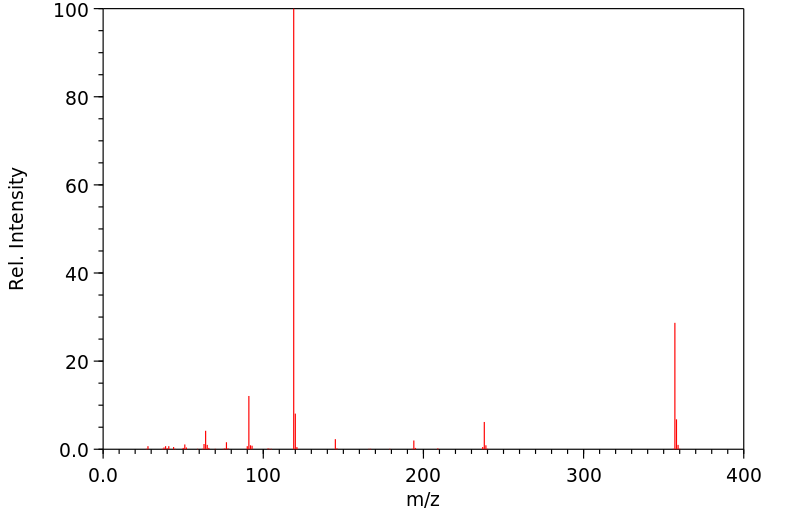
质谱MS
-
碳谱13CNMR
-
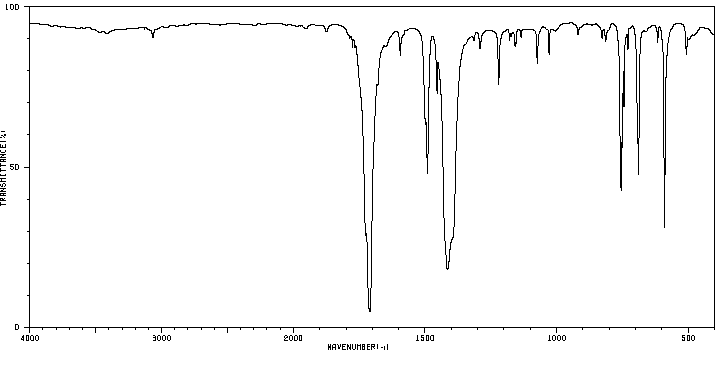
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿马诺嗪
阿特拉通
阿特拉津-乙氨基-15N1
阿特拉津-D5 同位素
阿特拉津
阿特拉嗪去异丙基-2-羟基
阿扎丙宗
达卡巴嗪相关物质B
败脂酸,丙-2-烯腈,苯乙烯
西草净亚砜
西草净
西玛津
螺拉秦
蜜勒胺
莠灭净
莠去津-特丁净混合物
莠去津-13C3
莠去津
草达津-2-羟基
草达津
苯酚,2-(4-氨基-6-乙氧基-1,3,5-三嗪-2-基)-
苯并呋喃,2-环丙基-
苯基-1,3,5-三嗪
苯嗪草酮-DESAMINO
苯嗪草酮
肼基氰尿酸盐
聚磷酸三聚氰胺
聚[[6-[(1,1,3,3-四甲基丁基)氨基]-1,3,5-三嗪-2,4-二基][(2,2,6,6-四甲基-4-哌啶基)亚氨基]-1,6-己二基[(2,2,6,6-四甲基-4-哌啶基)亚氨]]
聚(氧代-1,2-乙二氧基羰基-2,6-萘二基羰基)
羟硝基
美拉肼
美司钠EP杂质E
硫酸三聚氰胺
癸基-(二氯-[1,3,5]三嗪-2-基)-胺
甲氧丙净
甲基[2-(苯甲基氨基)-4-(4-氯苯基)-1,3-噻唑-5-基]乙酸酯
甲基6-甲基-1,2,3-三嗪-4-羧酸酯
甲基5-甲基-1,2,3-三嗪-4-羧酸酯
甲基-[1,2,4]噻嗪-3-基-胺
甲基(4Z)-4-(羟基亚胺)-2-甲基-4,5-二氢-1H-咪唑-1-羧酸酯
甲基(2E)-3-吖丙啶-1-基丙-2-烯酸酯
环氯胍硝酸盐
环氯胍
环己基三聚氰胺
环己基-(1-氧代-苯并[1,2,4]三嗪-3-基)-胺
环丙胺,N-[2-[(4-甲基苯基)硫代]乙基]-
环丙津-脱异丙基
环丙津-2-羟基
环丙津
环丙氨嗪-D4