特丁净 | 886-50-0
中文名称
特丁净
中文别名
2-甲硫基-4-乙氨基-6-特丁氨基-1,3,5-三嗪;2-特丁氨基-4-乙氨基-6-甲硫基-1,3,5-三嗪;去草净
英文名称
terbutryn
英文别名
N-(1,1-dimethylethyl)-N'-ethyl-6-(methylthio)-1,3,5-triazine-2,4-diamine;2-tert-butylamino-4-ethylamino-6-methylthio-1,3,5-triazine;N2-tert-butyl-N4-ethyl-6-methylthio-1,3,5-triazine-2,4-diamine;terbutryne;N2-tert-butyl-N4-ethyl-6-(methylsulfanyl)-1,3,5-triazine-2,4-diamine;2-N-tert-butyl-4-N-ethyl-6-methylsulfanyl-1,3,5-triazine-2,4-diamine;2-methylthio-4-ethylamino-6-tert-butylamino-1,3,5-triazine
CAS
886-50-0
化学式
C10H19N5S
mdl
MFCD00128025
分子量
241.36
InChiKey
IROINLKCQGIITA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:104-105°C
-
沸点:154-160°C
-
密度:1.45
-
闪点:2 °C
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
颜色/状态:WHITE, CRYSTALLINE
-
蒸汽压力:1.69X10-6 mm Hg at 25 °C
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
-
分解:When heated to decomposition it emits very toxic fumes of /nitrogen and sulfur oxides/.
-
腐蚀性:Noncorrosive
-
解离常数:pKa = 4.30 (weak base)
-
碰撞截面:161.23 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
-
保留指数:1902 ;1910 ;1906 ;1912 ;1935.4 ;1916.9 ;1906.2 ;1910.5 ;1940 ;1911.5 ;1940 ;1921.1 ;1910
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:16
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.7
-
拓扑面积:88
-
氢给体数:2
-
氢受体数:6
ADMET
代谢
Terbutryn ... was metabolized by both rats and goats after a single oral dose by one or more of the following pathways: S-demethylation, conversion of thiomethyl into hydroxyl, N-de-ethylation, oxidation of the terminal carbon of the ethyl group to a carboxylic acid, oxidation of a terminal carbon of the t-butyl group to an alcohol or a carboxylic acid, or conjugation with glucuronic acid.
来源:Hazardous Substances Data Bank (HSDB)
代谢
碳标记的特丁酯通过口服单次剂量给予大鼠和山羊。在长达72小时的间隔内收集尿液,并通过色谱程序分离葡萄糖苷酸后进行分析。分离并鉴定出的五种结合物包括:2-氨基-4-(叔丁氨基)-6-(S-葡萄糖苷酸基)-s-三嗪;2-(叔丁氨基)-4-乙氨基-6-(S-葡萄糖苷酸基)-s-三嗪;2-乙氨基-(2-甲基)葡萄糖苷酸基丙基)氨基-6-(S-甲硫基)-s-三嗪;2-氨基-4-(2-(1-葡萄糖苷酸基-2-甲基丙基)氨基)-6-甲硫基-s-三嗪;2-乙氨基-4-(2-(2-甲基丙烷-1-醇基)氨基)-6-(S-葡萄糖苷酸基)-s-三嗪。
Carbon-labeled terbutryn was admin as single oral doses to rats and goats. Urine was collected at intervals up to 72 hr and then analyzed ... after isolation of glucuronides by chromatographic procedures. Five conjugates isolated and identified were: 2-amino-4-(t-butylamino)-6-(S-glucuronyl)-s-triazine; 2-(t-butylamino)-4-ethylamino-6-(S-glucuronyl)-s-triazine; 2-ethyl-amino-(2-methyl)glucuronylpropyl)amino-6-(S-methylthio)-s-triazine; 2-amino-4-(2-(1-glucuronyl-2-methylpropyl)amino)-6-methylthio-s-triazine; 2-ethylamino-4-(2-(2-methyl propan-1-olyl)amino)-6-(S-glucuronyl)-s-triazine.
来源:Hazardous Substances Data Bank (HSDB)
代谢
在大鼠给药特布他林后,观察到的尿代谢物包括:2-羟基特布他林;2-氨基-4-羟基-6-叔丁氨基-s-三嗪;2-氨基-4-叔丁氨基-6-巯基-s-三嗪;两种S-葡萄糖苷酸和两种叔丁基-O-葡萄糖苷酸。其他代谢物是由以下一种或多种反应形成的:N-烷基氧化成醇或酸;S-脱甲基;N-脱乙基;以及二硫键形成。
After administration of terbutryne to rats, urinary metabolites observed ... included: 2-hydroxy terbutryne; 2-amino-4-hydroxy-6-t-butylamino-s-triazine; 2-amino-4-t-butylamino-6-mercapto-s-triazine; two S-glucuronides and two t-butyl-O-glucuronides. Other metabolites were formed by one or a combination of the following reactions: N-alkyl oxidation to alcohols or acids: S-demethylation; N-deethylation; and disulfide formation.
来源:Hazardous Substances Data Bank (HSDB)
代谢
从30到70岁接受肝脏切除手术的患者的肝脏中准备的微体,与6.3至1000微摩尔浓度的阿特拉津、特丁津、特丁灵或氨三嗪一起孵化,并分析了孵化混合物中的代谢物。这些化合物产生了一系列代谢物,表明发生了S-氧化、N-脱烷基化和侧链C-氧化。这些代谢物是通过显示双相动力学的过程形成的,第一相和第二相的米氏常数分别为1.4至20微摩尔和54至530微摩尔。将25微摩尔的阿特拉津、特丁津、氨三嗪或特丁灵与含有细胞色素P4501A2(CYP1A2)、细胞色素P4502A6、细胞色素P4502D6、细胞色素P4502C9、细胞色素P4502C19、细胞色素P4502E1或细胞色素P4503A4(CYP3A4)同种物的底物的人肝微体一起孵化。其他微体准备物在存在或不存在α-萘黄酮(aNF)、呋拉菲林、奎尼丁、磺胺苯唑、二乙基二硫代氨基甲酸酯、孕酮或酮康唑的情况下,与25或600微摩尔的S-三嗪一起孵化,这些抑制剂针对各种特定的细胞色素P450(P450)同种物,浓度是它们抑制常数的5到10倍。含有CYP1A2和CYP3A4底物的微体准备物与S-三嗪的代谢率显示出最好的相关性。只有aNF和呋拉菲林,CYP1A2的抑制剂,抑制了S-三嗪的代谢。将显示出高黄素含单加氧酶(FMO)活性的人肝微体准备物和纯化的重组人FMO-3与氨三嗪和特丁灵一起孵化。确定了这两种化合物亚砜化的程度。没有检测到显著的亚砜代谢物形成,这表明FMO系统没有参与人肝微体对S-三嗪的代谢。作者得出结论,这些结果清楚地确定了CYP1A2是参与人肝微体代谢S-三嗪的主要I相P450同种物。
Microsomes prepared from livers from 30 to 70 year old patients undergoing liver resection were incubated with 6.3 to 1,000 uM atrazine, terbuthylazine, terbutryne, or ametryne , and the incubation mixtures were analyzed for metabolites. The compounds produced a variety of metabolites indicative of S-oxidation, N-dealkylation, and side chain C-oxidation. The metabolites were formed by processes showing biphasic kinetics, Michaelis constants for the first and second phases varying from 1.4 to 20 uM and from 54 to 530 uM, respectively. Atrazine, terbuthylazine, ametryne, or terbutryne at 25 uM was incubated with human liver microsomes containing substrates for cytochrome-P4501A2 (CYP1A2), cytochrome-P4502A6, cytochrome-P4502D6, cytochrome-P4502C9, cytochrome-P4502C19, cytochrome-P4502E1, or cytochrome-P4503A4 (CYP3A4) isozymes. Other microsomal preparations were incubated with 25 or 600 uM of the S-triazines in the presence or absence of alpha-naphthoflavone (aNF), furafylline, quinidine, sulfaphenazole, diethyl-dithiocarbamate, gestodene, or ketoconazole, inhibitors of various specific cytochrome-P450 (P450) isozymes, at concentrations 5 to 10 times greater than their inhibition constants. Microsomal preparations containing substrates for CYP1A2 and CYP3A4 showed the best correlation with the rates of metabolism of the S-triazines. Only aNF and furafylline, inhibitors of CYP1A2, inhibited metabolism of the S-triazines. A human liver microsomal preparation with demonstrated high levels of flavin containing monooxygenase (FMO) activity and purified recombinant human FMO-3 were incubated with ametryne and terbutryne. The extent of sulfoxidation of the two compounds was determined. No significant formation of sulfoxide metabolites was detected, indicating that the FMO system was not involved in the metabolism of S- triazines by human liver microsomes. The authors conclude that these results clearly identify CYP1A2 as the major phase-I P450 isozyme that is involved in the metabolism of S-triazines by human liver microsomes.
来源:Hazardous Substances Data Bank (HSDB)
代谢
“Terbutryn 已知的代谢物包括 Terbutrynsulfoxide、2-[[4-(乙氨基)-6-甲基巯基-1,3,5-三嗪-2-基]氨基]-2-甲基-1-丙醇和 1-[[4-(叔丁氨基)-6-甲基巯基-1,3,5-三嗪-2-基]氨基]乙醇。”
Terbutryn has known human metabolites that include Terbutrynsulfoxide, 2-[[4-(Ethylamino)-6-methylsulfanyl-1,3,5-triazin-2-yl]amino]-2-methylpropan-1-ol, and 1-[[4-(tert-butylamino)-6-methylsulfanyl-1,3,5-triazin-2-yl]amino]ethanol.
来源:NORMAN Suspect List Exchange
毒理性
癌症分类:C组可能的人类致癌物
Cancer Classification: Group C Possible Human Carcinogen
来源:Hazardous Substances Data Bank (HSDB)
毒理性
神经毒素 - 其他中枢神经系统神经毒素
Neurotoxin - Other CNS neurotoxin
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
LC50 (大鼠) > 8000 mg/m³/4h
LC50 (rat) > 8,000 mg/m3/4h
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
/SRP:/ 基本治疗:建立专利气道(如有需要,使用口咽或鼻咽气道)。如有必要,进行吸痰。鼓励患者深呼吸。观察呼吸不足的迹象,如有必要,协助通气。通过非循环呼吸面罩以10至15升/分钟的速度给予氧气。监测肺水肿,如有必要,进行治疗……。监测休克并治疗,如有必要……。预见并治疗癫痫发作,如有必要……。对于眼睛污染,立即用水冲洗眼睛。在转运过程中,用0.9%的生理盐水(NS)持续冲洗每只眼睛……。不要使用催吐剂。对于摄入,如果患者能吞咽、有强烈的呕吐反射且不流口水,则用水冲洗口腔,并给予5毫升/千克,最多200毫升的水进行稀释……。/刺激物材料/
/SRP:/ Basic Treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Encourage patient to take deep breaths. Watch for signs of respiratory insufficiency and assist ventilations if necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . /Irritating materials/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 高级治疗:对于无意识、严重肺水肿或严重呼吸困难的病人,考虑进行口咽或鼻咽气管插管以控制气道。在上呼吸道阻塞的初期迹象出现时,可能需要尽早进行插管。使用气囊面罩装置的正压通气技术可能有益。考虑使用药物治疗肺水肿……监测心率和必要时治疗心律失常……开始静脉输注D5W /SRP: "保持开放",最低流量/。如果出现低血容量的迹象,使用0.9%生理盐水(NS)或乳酸钠林格氏液。对于伴有低血容量迹象的低血压,谨慎给予液体。注意液体过载的迹象……用地西泮或劳拉西泮治疗癫痫……使用丙美卡因氢氯化物协助眼部冲洗……/刺激性材料/
/SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Early intubation at the first sign of upper airway obstruction may be necessary. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Monitor cardiac rhythm and treat arrhythmias if necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... .Treat seizures with diazepam or lorazepam ... Use proparacaine hydrochloride to assist eye irrigation ... . /Irritating materials/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
They are efficiently absorbed from intestine, and presumably there is some absorption across the skin and lung. /Urea-, uracil- and triazine-based herbicides/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
通过叶子和根吸收。它似乎能迅速穿透叶子,减少雨水从叶子中冲刷。它通过木质部从根部和叶子向顶部运输,在顶端分生组织中积累。
Absorbed through both foliage and roots. It appears to penetrate foliage rapidly, minimizing removal from foliage by rain. /It is/ translocated acropetally through xylem from roots and foliage, accumulating in apical meristems.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在哺乳动物中,口服给药后,73-85%的药物以代谢形式在24小时内通过粪便排出。
In mammals, following oral admin, 73-85% is eliminated in metabolized form in feces within 24 hr.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险品标志:Xn,F,Xi,N
-
安全说明:S16,S26,S36/37,S60,S61
-
危险类别码:R36
-
WGK Germany:2
-
危险品运输编号:UN 3077 9/PG 3
-
RTECS号:XY4725000
-
海关编码:2933699012
-
包装等级:I; II; III
-
危险类别:6.1
-
储存条件:存储在阴凉干燥处,置于通风良好的地方,远离热源及不相容物质,并密封保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 特丁净
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 5)
急性毒性, 吸入 (类别 5)
皮肤刺激 (类别 3)
眼睛刺激 (类别 2A)
急性水生毒性 (类别 1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H303 吞咽可能有害。
H316 造成轻微皮肤刺激。
H319 造成严重眼刺激。
H333 吸入可能有害。
H400 对水生生物毒性极大。
警告申明
预防
P264 操作后彻底清洁皮肤。
P273 避免释放到环境中。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
响应
P304 + P312 如果吸入: 如感觉不适,呼救解毒中心或看医生。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P391 收集溢出物。
处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C10H19N5S
分子式
: 241.36 g/mol
分子量
组分 浓度或浓度范围
Terbutryn
-
化学文摘登记号(CAS 886-50-0
No.) 212-950-5
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 硫氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
一定要避免排放到周围环境中。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 104 °C
f) 沸点、初沸点和沸程
154 - 160 °C 在 0.08 hPa
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 3.74
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 2,045 mg/kg
半数致死浓度(LC50) 吸入 - 大鼠 - 4 h - > 8,000 mg/m3
半数致死剂量 (LD50) 经皮 - 兔子 - > 10,200 mg/kg
皮肤刺激或腐蚀
皮肤 - 兔子 - 轻度的皮肤刺激
眼睛刺激或腐蚀
眼睛 - 兔子 - 中度的眼睛刺激
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: XY4725000
模块 12. 生态学资料
12.1 生态毒性
对鱼类的毒性 半数致死浓度(LC50) - 其他鱼 - 3 mg/l - 96.0 h
对水蚤和其他水生无脊 半数效应浓度(EC50) - 大型蚤 (水蚤) - 7.1 mg/l - 48 h
椎动物的毒性
对藻类的毒性 半数效应浓度(EC50) - 近头状伪蹄形藻 (绿藻) - 2.4 - 2.9 mg/l - 96 h
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
对水生生物毒性极大。
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 3077 国际海运危规: 3077 国际空运危规: 3077
14.2 联合国运输名称
欧洲陆运危规: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Terbutryn)
国际海运危规: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Terbutryn)
国际空运危规: EnvironmeNTAlly hazardous subSTance, solid, n.o.s. (Terbutryn)
14.3 运输危险类别
欧洲陆运危规: 9 国际海运危规: 9 国际空运危规: 9
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 是 国际海运危规 国际空运危规: 是
海洋污染物(是/否): 是
14.6 对使用者的特别提醒
进一步信息
危险品独立包装,液体5升以上或固体5公斤以上,每个独立包装外和独立内包装合并后的外包装上都必须有EHS
标识 (根据欧洲 ADR 法规 2.2.9.1.10, IMDG 法规 2.10.3),
模块 16. 其他信息
进一步信息
版权所有:2012 Co. LLC. 公司。许可无限制纸张拷贝,仅限于内部使用。
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
制备方法与用途
简介
特丁净是一种内吸传导型除草剂,可用于芽前及芽后除草。在土壤中持效期为3至10周。它主要应用于冬小麦、大麦、高粱、向日葵、马铃薯、豌豆、大豆和花生等作物田,有效防除多年生裸麦草、黑麦草以及秋季萌发的繁缕、母菊、罂粟、看麦娘、马唐和狗尾草。
毒性特丁净对大鼠急性经口毒性为LD502400~2980毫克/千克,小鼠为5000毫克/千克。亚慢性毒理学研究中,大鼠和狗无作用剂量分别为每天50毫克/千克和40毫克/千克。对鸟类低毒,母鸡的LD50为4000毫克/千克。此外,它对鱼类有中等毒性。
化学性质特丁净是一种白色粉末,熔点104~105℃,在20℃时蒸气压为0.128×10-3帕。它易溶于异丙醇、二甲苯等有机溶剂,在水中溶解度为58毫克/升。正常条件下性质稳定,无腐蚀性。
用途特丁净属于均三氮苯类除草剂,具有内吸传导作用,是一种选择性的芽前和芽后除草剂。适用于冬小麦、大麦、高粱、向日葵、花生、大豆和豌豆等作物田防除多种杂草,特别是多年生裸麦草、黑麦草以及秋季萌发的繁缕、母菊、罂粟、看麦娘、马唐和狗尾草。
用于冬小麦和大麦时的有效成分剂量为20~25克/100平方米,而用于芽后玉米时则为10克/100平方米。
生产方法特丁净通过三聚氯氰与特丁胺反应生成2,4-二氯-6-特丁氨基-1,3,5-三嗪;然后与乙胺作用生成2-氯-4-乙氨基-6-特丁氨基-1,3,5-三嗪,最后与甲硫醇反应完成合成。
分类农药
毒性分级中毒
急性毒性口服 - 大鼠 LD50: 2472 毫克/千克; 小鼠 LD50: 4492 毫克/千克
刺激数据皮肤 - 兔子 380 毫克 轻度;眼睛 - 兔子 76 毫克 中度
可燃性危险特性燃烧时产生有毒的硫氧化物和氮氧化物气体
储运特性需存放在通风、低温、干燥处
灭火剂干粉、泡沫、砂土
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Deethylterbutryne 30125-65-6 C8H15N5S 213.31
反应信息
-
作为反应物:描述:参考文献:名称:MUIR, D. C. G.;PITZE, M.;BLOUW, A. P.;LOCKHART, W. L., WEED RES., 1981, 21, N 2, 59-70摘要:DOI:
-
作为产物:描述:特丁津 、 碳酸二甲酯 在 potassium tert-butylate 、 palladium diacetate 、 三苯基膦 、 potassium thioacetate 作用下, 以 二甲基亚砜 为溶剂, 以99%的产率得到特丁净参考文献:名称:钯催化的硫代甲基化通过三组分交叉偶联策略摘要:在本报告中,设计了掩蔽的无机硫和碳酸二甲酯的组合,以实现芳基氯的硫代甲基化交叉偶联。值得注意的是,这种强有力的策略实现了带有未保护核糖的核苷的硫代甲基化,具有后期偶联作用的含氯化物的药物以及具有多个杂原子和空间位阻的除草剂。而且,该方案实际上适用于具有较低催化负载和较高产率的多克级合成。DOI:10.1021/acs.orglett.8b02677
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] 3-[(HYDRAZONO)METHYL]-N-(TETRAZOL-5-YL)-BENZAMIDE AND 3-[(HYDRAZONO)METHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE DERIVATIVES AS HERBICIDES<br/>[FR] DÉRIVÉS DE 3-[(HYDRAZONO))MÉTHYL]-N-(TÉTRAZOL-5-YL)-BENZAMIDE ET DE 3-[(HYDRAZONO)MÉTHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE UTILISÉS EN TANT QU'HERBICIDES申请人:SYNGENTA CROP PROTECTION AG公开号:WO2021013969A1公开(公告)日:2021-01-28The present invention related to compounds of Formula (I): or an agronomically acceptable salt thereof, wherein Q, R2, R3, R4, R5 and R6 are as described herein. The invention further relates to compositions comprising said compounds, to methods of controlling weeds using said compositions, and to the use of compounds of Formula (I) as a herbicide.本发明涉及以下式(I)的化合物或其农业上可接受的盐,其中Q、R2、R3、R4、R5和R6如本文所述。该发明还涉及包含所述化合物的组合物,使用这些组合物控制杂草的方法,以及将式(I)的化合物用作除草剂的用途。
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
[EN] HERBICIDALLY ACTIVE HETEROARYL-S?BSTIT?TED CYCLIC DIONES OR DERIVATIVES THEREOF<br/>[FR] DIONES CYCLIQUES SUBSTITUÉES PAR HÉTÉROARYLE À ACTIVITÉ HERBICIDE OU DÉRIVÉS DE CELLES-CI申请人:SYNGENTA LTD公开号:WO2011012862A1公开(公告)日:2011-02-03The invention relates to a compound of formula (I), which is suitable for use as a herbicide wherein G is hydrogen or an agriculturally acceptable metal, sulfonium, ammonium or latentiating group; Q is a unsubstituted or substituted C3-C8 saturated or mono-unsaturated heterocyclyl containing at least one heteroatom selected from O, N and S, or Q is heteroaryl or substituted heteroaryl; m is 1, 2 or 3; and Het is an optionally substituted monocyclic or bicyclic heteroaromatic ring; and wherein the compound is optionally an agronomically acceptable salt thereof.
-
TRIAZOLE ACC INHIBITORS AND USES THEREOF
表征谱图
-
氢谱1HNMR
-
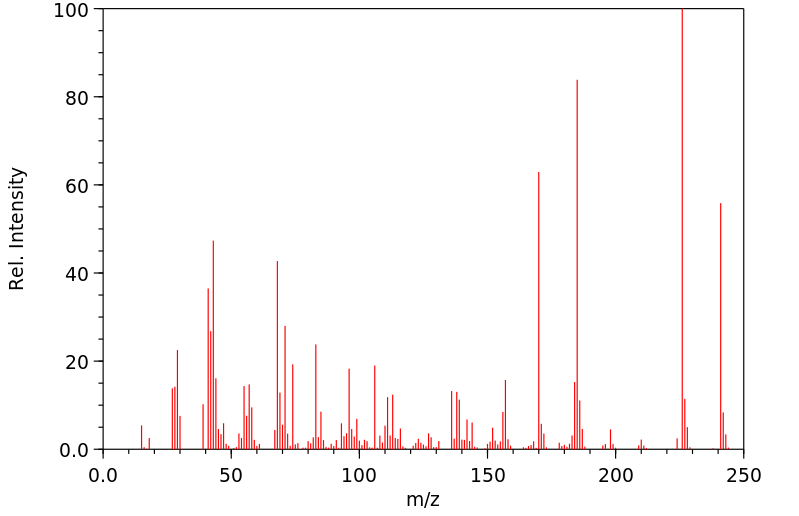
质谱MS
-
碳谱13CNMR
-
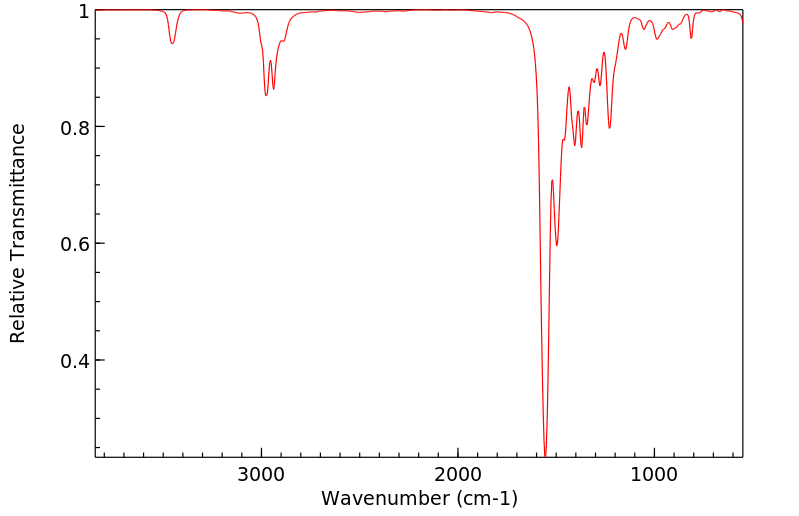
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿马诺嗪
阿特拉通
阿特拉津-乙氨基-15N1
阿特拉津-D5 同位素
阿特拉津
阿特拉嗪去异丙基-2-羟基
阿扎丙宗
达卡巴嗪相关物质B
败脂酸,丙-2-烯腈,苯乙烯
西草净亚砜
西草净
西玛津
螺拉秦
蜜勒胺
莠灭净
莠去津-特丁净混合物
莠去津-13C3
莠去津
草达津-2-羟基
草达津
苯酚,2-(4-氨基-6-乙氧基-1,3,5-三嗪-2-基)-
苯并呋喃,2-环丙基-
苯基-1,3,5-三嗪
苯嗪草酮-DESAMINO
苯嗪草酮
肼基氰尿酸盐
聚磷酸三聚氰胺
聚[[6-[(1,1,3,3-四甲基丁基)氨基]-1,3,5-三嗪-2,4-二基][(2,2,6,6-四甲基-4-哌啶基)亚氨基]-1,6-己二基[(2,2,6,6-四甲基-4-哌啶基)亚氨]]
聚(氧代-1,2-乙二氧基羰基-2,6-萘二基羰基)
羟硝基
美拉肼
美司钠EP杂质E
硫酸三聚氰胺
癸基-(二氯-[1,3,5]三嗪-2-基)-胺
甲氧丙净
甲基[2-(苯甲基氨基)-4-(4-氯苯基)-1,3-噻唑-5-基]乙酸酯
甲基6-甲基-1,2,3-三嗪-4-羧酸酯
甲基5-甲基-1,2,3-三嗪-4-羧酸酯
甲基-[1,2,4]噻嗪-3-基-胺
甲基(4Z)-4-(羟基亚胺)-2-甲基-4,5-二氢-1H-咪唑-1-羧酸酯
甲基(2E)-3-吖丙啶-1-基丙-2-烯酸酯
环氯胍硝酸盐
环氯胍
环己基三聚氰胺
环己基-(1-氧代-苯并[1,2,4]三嗪-3-基)-胺
环丙胺,N-[2-[(4-甲基苯基)硫代]乙基]-
环丙津-脱异丙基
环丙津-2-羟基
环丙津
环丙氨嗪-D4