1,1'-亚甲基二(3-甲基哌啶) | 68922-17-8
中文名称
1,1'-亚甲基二(3-甲基哌啶)
中文别名
1,1&prime-亚甲基双(3-甲基哌啶)
英文名称
di(3-methylpiperidin-1-yl)methane
英文别名
1,1'-methylenebis(3-methylpiperidine);bis(3-methylpiperidino)methane;3-methyl-1-[(3-methylpiperidin-1-yl)methyl]piperidine
CAS
68922-17-8
化学式
C13H26N2
mdl
MFCD00006518
分子量
210.363
InChiKey
KLFHRQOZJWCFOI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:160 °C/50 mmHg(lit.)
-
密度:0.887 g/mL at 25 °C(lit.)
-
闪点:113 °C
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:6.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
SDS
反应信息
-
作为反应物:描述:白杨素 、 1,1'-亚甲基二(3-甲基哌啶) 以 1,4-二氧六环 为溶剂, 以68%的产率得到5,7-dihydroxy-6,8-bis[(3-methylpiperidin-1-yl)methyl]-2-phenyl-4H-chromen-4-one参考文献:名称:Synthesis of Aminomethyl Derivatives of Chrysin摘要:通过醛胺的作用研究了金丝桃素的氨基甲基化反应。合成了6,8-双取代的金丝桃素衍生物。DOI:10.1007/s10600-013-0760-4
-
作为产物:参考文献:名称:Gem-diamines as highly active organocatalysts for carbon–carbon bond formation摘要:Diamines with neighbour nitrogen atoms have been used as base catalysts in the Knoevenagel condensation reaction between benzaldehyde and ethyl cyanoacetoacetate. The catalytic results show that a good basic catalyst requires a combination of two factors: high proton affinity and the ability to return the proton to the oxoanion intermediate. Computational chemistry calculations show this by characterizing the reactants, products, and transition states and by calculating the activation energies of the different reaction steps. A diamine, di(3-methylpiperidine)methane (B), has been found with a higher catalytic activity than DMAN despite its lower proton affinity, demonstrating that not only the proton affinity, but also the steric ability to abstract the protons, are important in explaining the catalytic results. (c) 2006 Elsevier Inc. All rights reserved.DOI:10.1016/j.jcat.2006.11.029
-
作为试剂:描述:参考文献:名称:Gem-diamines as highly active organocatalysts for carbon–carbon bond formation摘要:Diamines with neighbour nitrogen atoms have been used as base catalysts in the Knoevenagel condensation reaction between benzaldehyde and ethyl cyanoacetoacetate. The catalytic results show that a good basic catalyst requires a combination of two factors: high proton affinity and the ability to return the proton to the oxoanion intermediate. Computational chemistry calculations show this by characterizing the reactants, products, and transition states and by calculating the activation energies of the different reaction steps. A diamine, di(3-methylpiperidine)methane (B), has been found with a higher catalytic activity than DMAN despite its lower proton affinity, demonstrating that not only the proton affinity, but also the steric ability to abstract the protons, are important in explaining the catalytic results. (c) 2006 Elsevier Inc. All rights reserved.DOI:10.1016/j.jcat.2006.11.029
文献信息
-
[EN] PROCESS FOR PREPARING 1,2,3,9-TETRAHYDRO-9-METHYL-3-[(2-METHYL-1H-IMIDAZOLE-1-YL)METHYL]-4H-CARBAZOL-4-ONE OR ITS SALT<br/>[FR] PROCEDE DE PREPARATION DE 1,2,3,9-TETRAHYDRO-9-METHYL-3-[(2-METHYL-1H-IMIDAZOLE-1-YL)METHYL]-4H-CARBAZOL-4-ONE OU D'UN SEL DE CELUI-CI申请人:YUHAN CORP公开号:WO2005037822A1公开(公告)日:2005-04-281,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]- 4H-carbazol-4-one or its salt is prepared in high yield by reacting a compound of formula 2 with a compound of formula 3 and a compound of formula 4 in the presence of an acid, an alkylsilylhalide compound or an acylhalide compound, in an solvent, and thus, such an inventive process can be favorably applied to a large-scale mass production thereof.
-
Modified Coumarins. 11. Synthesis and Biological Activity of Mannich Bases of Substituted 1,3-Dihydrocyclopenta[c]chromen-4-ones作者:Ya. L. Garazd、T. N. Panteleimonova、M. M. Garazd、V. P. KhilyaDOI:10.1023/b:conc.0000003410.74701.dc日期:2003.7Mannich bases containing the dialkylaminomethyl group in the 6- and 8-positions of 2,3-di-hydrocyclopenta[c]chromen-4-ones were prepared by condensation of 7- and 9-hydroxy-2,3-dihydrocyclopenta[c]chromen-4-ones with substituted 1,1-diaminomethanes. The effects of 8-chloro-7-hydroxy-6-(1-pyrrolidinylmethyl)-2,3-dihydrocyclopenta[c]chromen-4-one and 8-chloro-7-hydroxy-6-(morpholinomethyl)-2,3-dihyd曼尼希碱在 2,3-di-hydrocyclopenta[c]chromen-4-ones 的 6-和 8-位含有二烷基氨基甲基,是通过 7-和 9-羟基-2,3-dihydrocyclopenta[c] 缩合制备的chromen-4-ones 与取代的 1,1-二氨基甲烷。8-chloro-7-hydroxy-6-(1-pyrrolidinylmethyl)-2,3-dihydrocyclopenta[c]chromen-4-one 和 8-chloro-7-hydroxy-6-(morpholinomethyl)-2,3 的作用-dihydrocyclopenta[c]chromen-4-one 对中枢和外周神经系统进行了定义,并能够预测镇静和精神安定特性的存在。
-
Synthesis of aminomethyl derivatives of sophoricoside作者:S. P. Bondarenko、M. S. Frasinyuk、V. P. KhilyaDOI:10.1007/s10600-012-0151-2日期:2012.3Aminomethylation of the natural isoflavonoid sophoricoside (genistein-4-O-β-D-glucoside) was studied. It was shown that the most convenient method for performing the aminomethylation was the use of aminals. 6,8-bis-Substituted derivatives of sophoricoside were synthesized.
-
Suppression of the evolution of hydrogen sulfide gases from petroleum residua申请人:PETROLITE CORPORATION公开号:EP0383499A3公开(公告)日:1990-10-03Hydrogen sulfide gas evolution during storage or transport of petroleum residua are suppressed by the incorporation of an effective amount of a diamine of the formula wherein R₁, R₂, R₃, and R₄ are independently an alkyl radical containing 1 to 14 carbon atoms, (CH₂)n-OR₆ or cycloalkyl containing 5 or 6 carbon atoms and R₅ is hydrogen or methyl. R₁ and R₂ and R₃ and R₄ can be alkylene groups joined together with their respective adjacent N to form a heterocyclic ring. R₆ is hydrogen or an alkyl radical having 1 to 5 carbon atoms and n is an integer of 1 to 5.在石油残渣的储存或运输过程中,加入一定量的以下公式中R₁、R₂、R₃和R₄分别独立地表示含有1至14个碳原子的烷基基团,(CH₂)n-OR₆或含有5或6个碳原子的环烷基,而R₅为氢或甲基的二胺化合物,可以抑制氢硫酸气体的生成。其中,R₁和R₂以及R₃和R₄可以是相邻的氮原子结合在一起的亚烷基基团,形成杂环环。R₆为氢或具有1至5个碳原子的烷基基团,n为1至5的整数。
-
[EN] PRODUCTION METHOD OF FLUOROETHERCARBOXYLIC ACID FLUORIDE AND FLUOROETHERCARBOXYLIC ACID<br/>[FR] PROCÉDÉ DE PRODUCTION DE FLUORURE D'ACIDE FLUOROÉTHERCARBOXYLIQUE ET D'ACIDE FLUOROÉTHERCARBOXYLIQUE申请人:DAIKIN IND LTD公开号:WO2010038902A1公开(公告)日:2010-04-08The present invention is a production method of a fluoroethercarboxylic acid fluoride, comprising the step of selectively producing a fluoroethercarboxylic acid fluoride represented by the formula (I): CF3O(CF(CF3)CF2O)CF(CF3)COF (I) by reacting hexafluoropropylene oxide with carbonyl fluoride in a solvent at a temperature of -30 to 40 °C in the presence of a catalyst, wherein the catalyst comprises at least one species selected from the group consisting of metal fluorides, tertiary amines, tertiary diamines and tetraalkylammonium salts, and the solvent is an aprotic polar solvent.
表征谱图
-
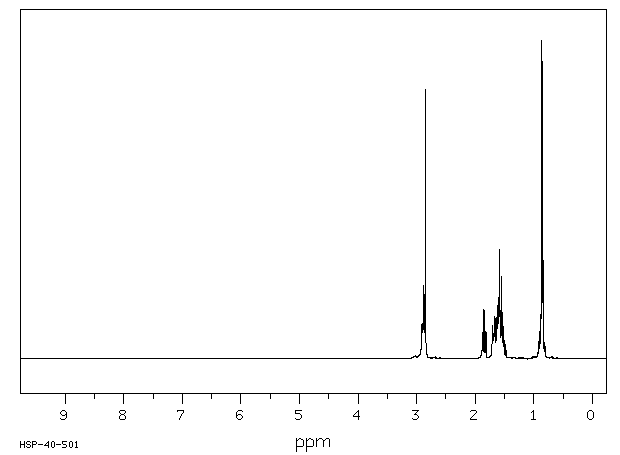
氢谱1HNMR
-
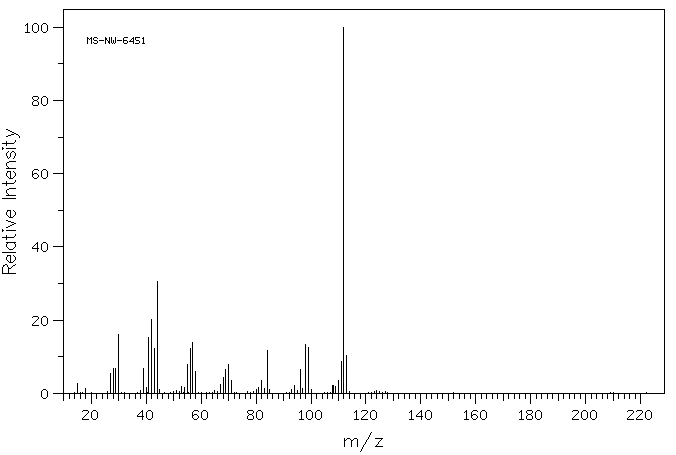
质谱MS
-
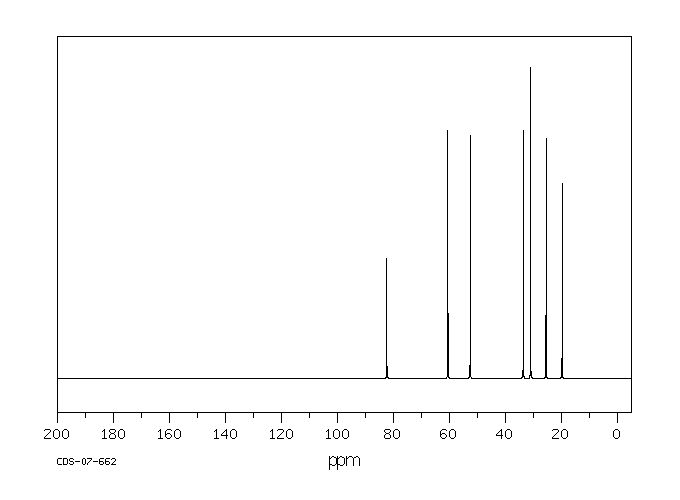
碳谱13CNMR
-
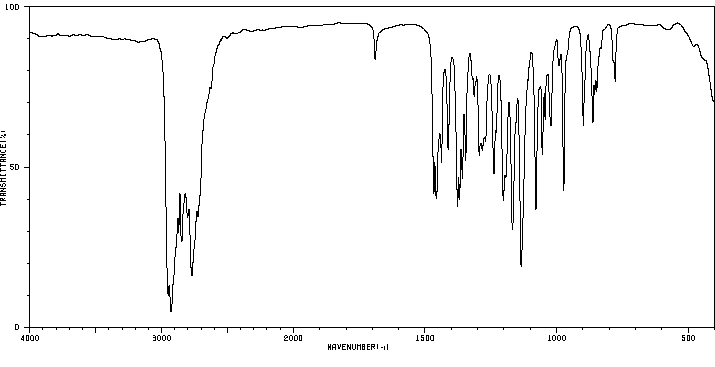
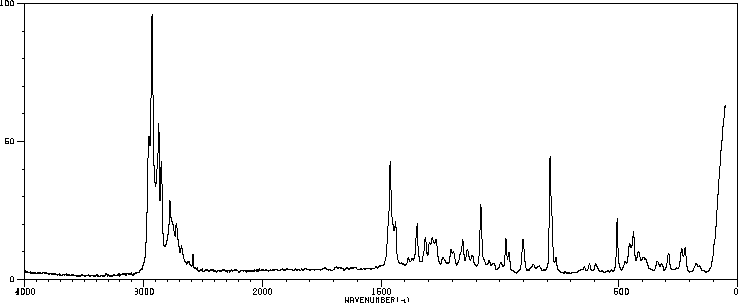
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺