3,4-二氯-1,2-萘二酮 | 18398-36-2
中文名称
3,4-二氯-1,2-萘二酮
中文别名
——
英文名称
3,4-dichloro-[1,2]naphthoquinone
英文别名
3,4-Dichlor-[1,2]naphthochinon;3,4-Dichlor-naphthochinon-(1,2);3,4-dichloro-1,2-naphthalenedione;3,4-Dichloro-1,2-naphthoquinone;3,4-dichloronaphthalene-1,2-dione
CAS
18398-36-2
化学式
C10H4Cl2O2
mdl
——
分子量
227.047
InChiKey
JDQAAXMSEQPHAM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:184°C
-
沸点:325.12°C (rough estimate)
-
密度:1.4057 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:14
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2914700090
SDS
上下游信息
反应信息
-
作为反应物:描述:3,4-二氯-1,2-萘二酮 在 calcium chloride 作用下, 生成 2-Trichloracetyl-benzoesaeure参考文献:名称:Zincke; Schmidt, Chemische Berichte, 1894, vol. 27, p. 744摘要:DOI:
-
作为产物:描述:参考文献:名称:Zincke, Chemische Berichte, 1886, vol. 19, p. 2499摘要:DOI:
文献信息
-
The reactions of some ortho-naphthoquinones with 2,3-dimethylbutadiene作者:M. F. Ansell、R. A. MurrayDOI:10.1039/j39710001429日期:——The reactions of dimethylbutadiene with 1,2-naphthoquinones are shown to involve either addition to the 3,4-double bond [substituents 3-chloro-, 4-cyano-, 3-methoxycarbonyl, 3-nitro-, and 3-methoxy-(partially)] or to the 2-carbonyl group [substituents 4-chloro-, 4-bromo-, 3,4-dichloro-, 3-methoxy-(partially)]. The adduct from 3-chloro-1,2-naphthoquinone is shown to eliminate hydrogen chloride to yield
-
Diazobenzo[a]fluorene derivatives as visible photosensitizers for free radical polymerization作者:Karolina Podemska、Radosław Podsiadły、Agnieszka Marzena Szymczak、Jolanta SokołowskaDOI:10.1016/j.dyepig.2011.12.001日期:2012.7energy change for electron transfer from the diazobenzo[a]fluorene dyes to the electron donors/acceptors. The kinetic studies of the photoinitiated polymerization of trimethylolpropane triacrylate (TMPTA) using electron donors, such as phenylthioacetic acid, phenoxyacetic acid, N-phenylglycine and ethyl 4-N,N-dimethylaminobenzoate, and electron acceptors, such as 1-methoxy-4-phenylpyridinium tetrafluoroborate已经合成了几种含有重氮苯并[a]芴部分的苯并吩嗪染料,并通过1 H NMR光谱和质谱(CI MS)对其进行了表征。检查了这些染料的光谱和电化学性质。这些化合物被评估为自由基聚合的潜在光吸收发色团。基于从重氮苯并[a]芴染料到电子供体/受体的电子转移的自由能变化,讨论了结果。使用电子给体如苯硫基乙酸,苯氧乙酸,N-苯基甘氨酸和乙基4- N,N的光引发聚合三丙烯酸三羟甲基丙烷酯(TMPTA)的动力学研究-二甲基氨基苯甲酸酯和电子受体,例如四氟硼酸1-甲氧基-4-苯基吡啶鎓和六氟磷酸1-乙氧基-2-甲基吡啶鎓,这些染料是可见光下自由基聚合的有效光引发剂。化学结构中存在的重原子可能会导致染料内激发三重态,从而促进这些态的电子转移。
-
Zincke; Schmunk, Justus Liebigs Annalen der Chemie, 1890, vol. 257, p. 145作者:Zincke、SchmunkDOI:——日期:——
-
Antiprotozoal quinones. II. Synthesis of 4-amino-1,2-naphthoquinones and related compounds as potential antimalarials作者:F. J. Bullock、J. F. Tweedie、D. D. McRitchie、M. A. TuckerDOI:10.1021/jm00295a025日期:1970.1
-
Zincke; Schuetz, Chemische Berichte, 1912, vol. 45, p. 644作者:Zincke、SchuetzDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
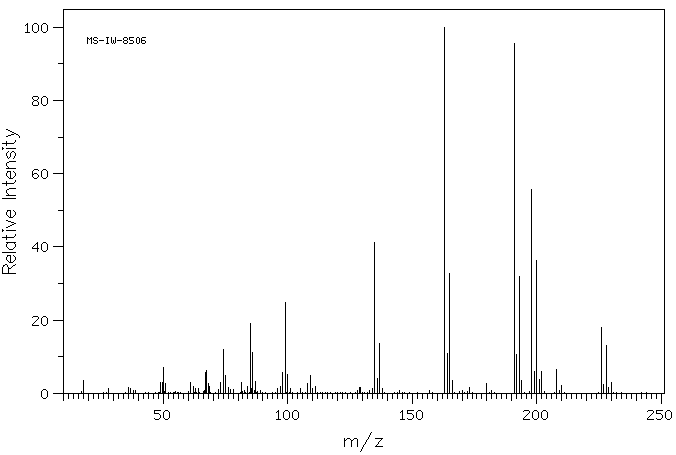
质谱MS
-
碳谱13CNMR
-
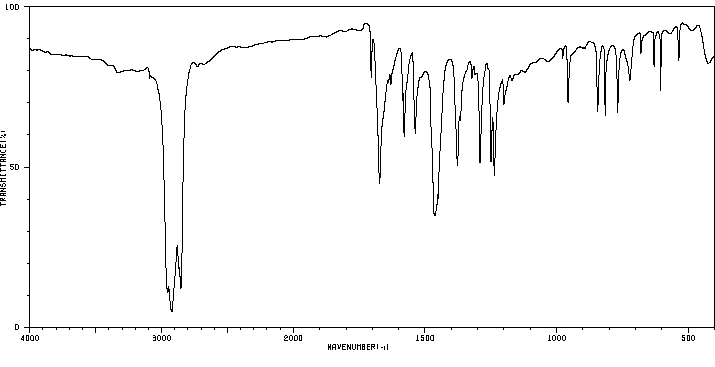
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮