1-吡啶甲酯 | 1530-87-6
中文名称
1-吡啶甲酯
中文别名
1-哌啶羰腈
英文名称
1-piperidinonitrile
英文别名
1-piperidinylcarbonnitrile;1-piperidinecarbonitrile;piperidine-1-carbonitrile
CAS
1530-87-6
化学式
C6H10N2
mdl
——
分子量
110.159
InChiKey
NVPICXQHSYQKGM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:102 °C10 mm Hg(lit.)
-
密度:0.951 g/mL at 25 °C(lit.)
-
闪点:207 °F
-
最大波长(λmax):214nm(MeOH)(lit.)
-
稳定性/保质期:
避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:27
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2933399090
-
包装等级:III
-
危险类别:6.1(b)
-
WGK Germany:3
-
危险品运输编号:UN 3276
-
储存条件:密封储存,存放在阴凉干燥的库房中,并远离火源。
SDS
| Name: | 1-Piperidinecarbonitrile 97+% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 1530-87-6 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1530-87-6 | 1-Piperidinecarbonitrile | 97+ | 216-233-8 |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. Harmful if absorbed through the skin. May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May cause cardiac disturbances. May cause central nervous system depression. Metabolism may release cyanide, which may result in headache, dizziness, weakness, collapse, unconsciousness and possible death.
Inhalation:
Causes respiratory tract irritation. May cause cardiac abnormalities. Can produce delayed pulmonary edema. May be metabolized to cyanide which in turns act by inhibiting cytochrome oxidase impairing cellular respiration. Inhalation at high concentrations may cause CNS depression and asphixiation.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Effects may be delayed. Chronic exposure to cyanide solutions may lead to the development of a "cyanide" rash, characterized by itching, and by macular, papular, and vesicular eruptions, and may be accompanied by secondary infections.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively. May be partially metabolized to cyanide in the body.
Antidote: Always have a cyanide antidote kit on hand when working with cyanide compounds. Get medical advice to use.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air.
They can spread along the ground and collect in low or confined areas. Containers may explode when heated. Combustible material; may burn but does not ignite readily. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Do NOT get water inside containers. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1530-87-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear almost colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 102 deg C @ 10.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: 97 deg C ( 206.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9510g/cm3
Molecular Formula:
Molecular Weight: 110.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong acids, strong bases, strong oxidizing agents, strong reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1530-87-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Piperidinecarbonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 1530-87-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1530-87-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1530-87-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:亚膦酸基钌(II)和(II)配合物催化水合氰胺:范围和机理的见解摘要:通过使用水合相应的氰胺R 1 R 2 NC N成功地完成了多种脲R 1 R 2 NC(O)NH 2的合成(R 1和R 2 =烷基,芳基或H; 26个实例)的三价膦酸基配合物[的MC1 2(η 6 - p -cymene)(PME 2 OH)](M =茹(1),锇(2))作为催化剂。反应在温和的条件下(40-70°C)干净进行,没有任何添加剂,使用低金属负载量(1 mol%)和水作为唯一溶剂。在几乎所有情况下,complex配合物2的反应活性都比钌配合物1高。另外,对于两种催化剂,氰酰胺底物水合所观察到的反应速率明显快于涉及传统脂族和芳族腈的反应速率。计算研究使我们能够合理化所有这些趋势。因此,计算表明存在直接与碳原子相连的氮原子当与金属中心配位时,N键通过感应效应使腈碳电子减少,从而促进次膦酸配体的OH基团对该碳的分子内亲核攻击。另一方面,Os对Ru的较高反应性似乎与初始金属环上较低的环应DOI:10.1039/d0cy00523a
-
作为产物:描述:参考文献:名称:Schroth, W.; Kluge, H.; Frach, R., Journal fur praktische Chemie (Leipzig 1954), 1983, vol. 325, # 5, p. 787 - 802摘要:DOI:
-
作为试剂:参考文献:名称:1,3-二氨基-6,7-二甲氧基异喹啉衍生物作为潜在的α1-肾上腺素受体拮抗剂。摘要:用LDA处理2-甲基-4,5-二甲氧基苄腈(3),然后与N,N-二取代的氰胺反应,得到一系列的1,3-二氨基-6,7-二甲氧基异喹啉(2),对其进行评估α-肾上腺素受体结合亲和力和降压活性。1-氨基-3-(二甲基氨基)-6,7-二甲氧基异喹啉(4)对α1-肾上腺素受体没有明显的亲和力(Ki远大于10(-6)M),而相应的3-(2-呋喃基哌嗪- 1-yl)类似物(8; Ki = 1.6 X 10(-7)M)的效力比哌唑嗪低约1000倍。pKa数据显示,在生理pH值下会发生4的N-2质子化(34%)(pKa = 7.1),这与8.HCl的X射线晶体学分析一致。质子化4与相应的喹啉和喹唑啉阳离子质子化后的正电荷分布的比较证实,N-1质子化是这些杂环核有效结合至α1-肾上腺素受体所必需的。计算机辅助比较8和哌唑嗪的X射线结构表明,α1-肾上腺素受体结合能的4.0 kcal / mol差异主要是由于DOI:10.1021/jm00400a026
文献信息
-
Oxidative cleavage of the CN bond during singlet oxygenations of amidoximates作者:Nüket Öcal、Ihsan ErdenDOI:10.1016/s0040-4039(01)00887-5日期:2001.7Amidoximes are inert toward singlet oxygen (1O2), however, the photooxygenation of amidoximate anions proceeds smoothly and in high yield to give mixtures of amides and nitriles. The mechanism of these reactions appears to involve carbonyl oxide intermediates. The oxidative cleavage of amidoximates closely resembles the results obtained from nitric oxide synthase (NOS) oxidations of N-hydroxyarginine
-
Nickelocene as an Air- and Moisture-Tolerant Precatalyst in the Regioselective Synthesis of Multisubstituted Pyridines作者:Il Young Cho、Woo Gyum Kim、Ji Hwan Jeon、Jeong Woo Lee、Jeong Kon Seo、Jongcheol Seo、Sung You HongDOI:10.1021/acs.joc.1c00577日期:2021.7.16Ni(COD)2-catalyzed cycloaddition reactions to access pyridines have been extensively studied. However, this catalyst typically requires drying procedures and inert-atmosphere techniques for the reactions. Herein, we report operationally simple nickel(0) catalysis to access substituted pyridines from various nitriles and 1,6-diynes without the aid of air-free techniques. The Ni-Xantphos-based catalytic
-
[EN] PYRIDIN- 2 -AMIDES USEFUL AS CB2 AGONISTS<br/>[FR] PYRIDIN-2-AMIDES UTILES COMME AGONISTES DE CB2申请人:HOFFMANN LA ROCHE公开号:WO2012168350A1公开(公告)日:2012-12-13The invention relates to a compound of formula (I) wherein R1 to R4 are defined as in the description and in the claims. The compound of formula (I) can be used as a medicament.本发明涉及式(I)的化合物,其中R1至R4的定义如描述和权利要求中所述。式(I)的化合物可以用作药物。
-
[EN] DIMERIC PIPERIDINE DERIVATIVES<br/>[FR] DERIVES DE PIPERIDINE DIMERES申请人:JANSSEN PHARMACEUTICA NV公开号:WO2006008260A1公开(公告)日:2006-01-26The compounds of the following formula (I) : the N-oxide forms, the pharmaceutically acceptable addition salts and the stereochemically isomeric forms thereof, useful in the treatment of neurodegenerative mediated disorders.以下公式(I)的化合物:N-氧化物形式,药用可接受的添加盐及其立体异构形式,在治疗神经退行性媒介紊乱中具有用途。
-
Peracid oxidation of an N-hydroxyguanidine compound: a chemical model for the oxidation of N.omega.-hydroxy-L-arginine by nitric oxide synthase作者:Jon M. Fukuto、Dennis J. Stuehr、Paul L. Feldman、Michael P. Bova、Patrick WongDOI:10.1021/jm00070a010日期:1993.9enzymes called the nitric oxide synthases (NOS) to generate citrulline and, presumably, nitric oxide (.NO). N-Hydroxylation of a guanidinium nitrogen of arginine to generate N-hydroxyarginine (NOHA) has been shown to be a step in the biosynthesis of .NO. In an effort to elucidate the mechanism by which further oxidation of NOHA occurs, the oxidation of a model N-hydroxyguanidine compound by several
表征谱图
-
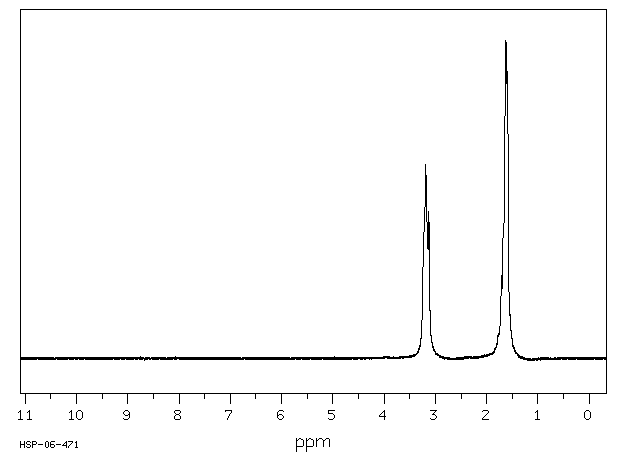
氢谱1HNMR
-
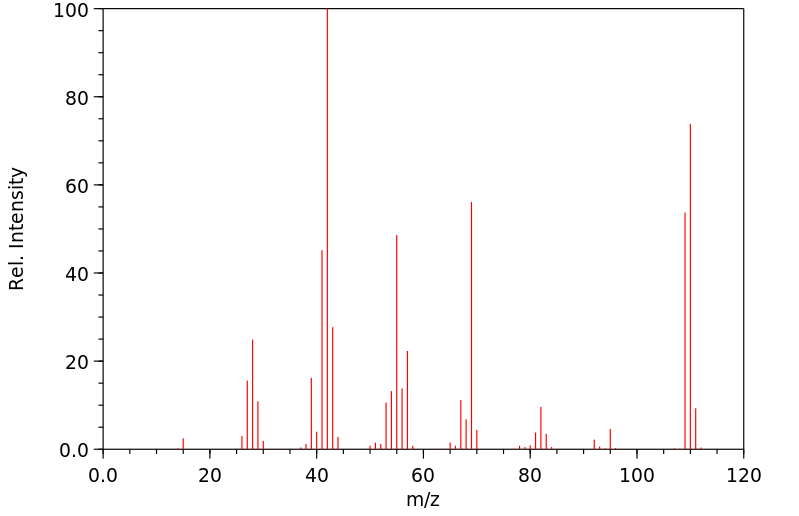
质谱MS
-
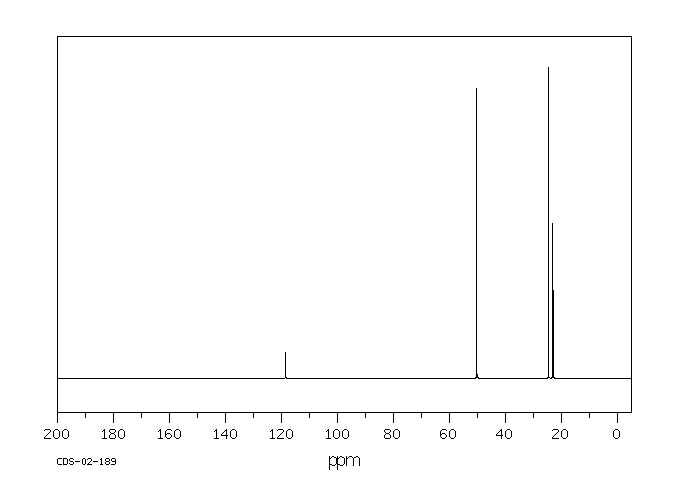
碳谱13CNMR
-
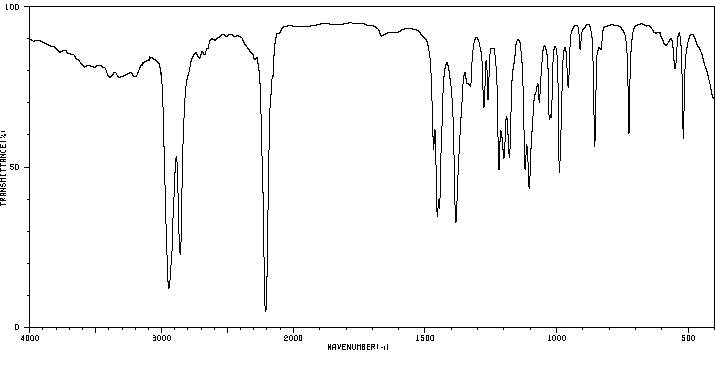
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺