四丙基锡 | 2176-98-9
分子结构分类
中文名称
四丙基锡
中文别名
四正丙基锡
英文名称
tetrapropylstannane
英文别名
tetrapropyltin;tetra-n-propyltin
CAS
2176-98-9
化学式
C12H28Sn
mdl
MFCD00015196
分子量
291.064
InChiKey
OIQCWAIEHVRCCG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-109℃
-
沸点:222°C
-
密度:1,107 g/cm3
-
闪点:222°C
-
溶解度:可溶于氯仿、乙酸乙酯(微量)、甲醇(微量)
-
暴露限值:ACGIH: TWA 0.1 mg/m3; STEL 0.2 mg/m3 (Skin)NIOSH: IDLH 25 mg/m3; TWA 0.1 mg/m3
-
保留指数:1325;1326;1329;1327
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):6.34
-
重原子数:13
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:6.1
-
安全说明:S26,S27,S36/37/39
-
危险类别码:R23/24/25
-
海关编码:2931900090
-
危险品运输编号:UN 2788
-
储存条件:储存地点应远离氧化剂,并确保容器密封、存放在坚固的容器中。请将其存放在阴凉干燥处。
SDS
Section 1: Product Identification
Chemical Name: Tetra-n-propyltin, min. 97%
CAS Registry Number: 2176-98-9
Formula: Sn(C3H7)4
EINECS Number: 218-536-0
Chemical Family: organotin compound
Synonym: Tin, tetrapropyl-, Tetrapropylstannane
Section 2: Composition and Information on Ingredients
Ingredient CAS Number Percent ACGIH (TWA) OSHA (PEL)
Title Compound 2176-98-9 100% 0.1mg/m3 0.1mg/m3
Section 3: Hazards Identification
Emergency Overview: Irritating to the respiratory tract, skin and eyes. May be harmful if swallowed.
Primary Routes of Exposure: Ingestion, inhalation, skin, eyes
Eye Contact: Causes severe irritation of the eyes.
Causes slight to mild irritation of the skin. Prolonged contact may dry the skin and lead to rashes or more
Skin Contact:
severe irritation.
Inhalation: Irritating to skin, eyes and respiratory tract.
Delayed and permanent psycho-neurological disturbances; impairment of vision, unsteadiness, nausea and
Ingestion:
vomiting.
Irritating to skin, eyes and mucous membranes. Ingestion of certain organotin compounds may cause delayed
Acute Health Affects:
poisoning (4 days) with cerebral edema causing damage to the central nervous system.
Repeated exposure to certain organic tin compounds may cause problems with vision, skin, respiratory
Chronic Health Affects:
system, central nervous system, liver, kidneys, urinary tract, and blood.
NTP: No
IARC: No
OSHA: No
SECTION 4: First Aid Measures
Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need
Eye Exposure:
assistance in keeping their eye lids open. Get immediate medical attention.
Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
Skin Exposure:
irritation persists.
Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty
Inhalation:
in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance.
Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
Ingestion:
vomiting only if directed by medical personnel.
SECTION 5: Fire Fighting Measures
Flash Point: no data
Autoignition Temperature: no data
Explosion Limits: no data
Extinguishing Medium: carbon dioxide, foam or dry powder
Fire fighters should be equipped with a NIOSH approved positive pressure self-contained breathing apparatus
Special Fire Fighting Procedures:
and full protective clothing.
Hazardous Combustion and carbon monoxide, carbon dioxide, soot, organic fumes and tin compounds.
Decomposion Products:
Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards.
SECTION 6: Accidental Release Measures
Small spills can be mixed with vermiculite, ground limestone, sodium carbonate or other suitable
Spill and Leak Procedures:
noncombustible adsorbent and swept up.
SECTION 7: Handling and Storage
Handling and Storage: Store in a cool, dry, well ventilated area away from heat and direct sunlight. Keep containers tightly sealed.
SECTION 8: Exposure Controls and Personal Protection
Eye Protection: Always wear approved safety glasses when handling a chemical substance in the laboratory.
Skin Protection: Wear protective clothing and gloves. Consult with glove manufacturer to determine the proper type of glove.
Ventilation: Work with this product in a well-ventilated area, preferably a fume hood.
If ventilation is not available a respirator should be worn. The use of respirators requires a Respirator
Respirator:
Protection Program to be in compliance with 29 CFR 1910.134.
Ventilation: Work with this product in a well-ventilated area, preferably a fume hood.
Additional Protection: No additional protection required.
SECTION 9: Physical and Chemical Properties
Color and Form: colorless liq.
Molecular Weight: 291.05
Melting Point: no data
Boiling Point: 222°C
Vapor Pressure: no data
Specific Gravity: 1.1065(20°)
Odor: not determined
Solubility in Water: insoluble
SECTION 10: Stability and Reactivity
Stability: air and moisture stable
Hazardous Polymerization: no hazardous polymerization
Conditions to Avoid: none
Incompatibility: strong oxidizing agents and halogens
Decomposition Products: carbon monoxide, carbon dioxide, tin oxide, and organic fumes.
SECTION 11: Toxicological Information
RTECS Data: Parenteral (mouse); LDLo: 73 mg/kg.
Carcinogenic Effects: No data available
Mutagenic Effects: No data available
Tetratogenic Effects: No data available
SECTION 12: Ecological Information
Ecological Information: No information available
SECTION 13: Disposal Considerations
Disposal: Dispose of according to federal, state, and local regulations.
SECTION 14: Transportation
Shipping Name (CFR): Non-hazardous
Hazard Class (CFR): NA
Additional Hazard Class (CFR): NA
Packaging Group (CFR): NA
UN ID Number (CFR): NA
Shipping Name (IATA): Non-hazardous
Hazard Class (IATA): NA
Additional Hazard Class (IATA): NA
Packaging Group (IATA): NA
UN ID Number (IATA): NA
SECTION 15: Regulatory Information
TSCA: Not listed in the TSCA inventory.
SARA (Title 313): Title compound not listed.
Second Ingredient: none
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三正丙基氯化锡 tripropyltin chloride 2279-76-7 C9H21ClSn 283.429 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Di-n-butyl-di-n-propylzinn 3634-61-5 C14H32Sn 319.118 三正丙基氯化锡 tripropyltin chloride 2279-76-7 C9H21ClSn 283.429 三丙基锡 tripropyl stannane 761-44-4 C9H22Sn 248.984 —— tributyl(vinyl)tin 20474-38-8 C11H24Sn 275.022
反应信息
-
作为反应物:参考文献:名称:Systematische untersuchungen尤伯杯DAS verhalten冯organozinn-verbindungengegenüberflüssigemschwefeldioxid:一Reaktionen冯tetraalkylstannanen MITflüssigemSO 2摘要:根据水的存在,反应温度和温度,研究了将SO 2插入四烷基锡化合物R 4 Sn(R = CH 3,C 2 H 5,nC 3 H 7,iC 3 H 7,nC 4 H 9)中的情况。反应时间。根据eqns。(1)–(5),反应产物R 3 SnO 2 SR,R 2 Sn(O 2 SR)2,(R 3 Sn)2 SO 4,R 2 SnSO 4和R 2分离出SnSO 3。尤其; 在低温(<0°)下,建立了teraorganostannanes对SO 2降低反应性的以下顺序:R = C 2 H 5 > CH 3 > nC 3 H 7 > nC 4 H 9 > iC 3 H 7DOI:10.1016/s0022-328x(00)81360-1
-
作为产物:描述:参考文献:名称:Pfeiffer, Zeitschrift fur Anorganische und Allgemeine Chemie, 1910, vol. 68, p. 112摘要:DOI:
-
作为试剂:描述:参考文献:名称:Uglova,E.V. et al., Journal of Organic Chemistry USSR (English Translation), 1975, vol. 11, p. 1 - 4摘要:DOI:
文献信息
-
Substitution at saturated carbon. Part II. Kinetics and mechanism of the electrophilic substitution of several tetra-alkyltins by mercuric iodide in solvent 96% methanol–4% water作者:M. H. Abraham、T. R. SpaldingDOI:10.1039/j19690000399日期:——The substitution of tetra-alkyltins by mercuric iodide in solvent 96% methanol–4% water proceeds by the rate-determining bimolecular reaction R4Sn + Hgl2→ RHgl + R3Snl, followed by the rapid reversible reaction R3Snl + Hgl2⇌ R3Sn++ Hgl3–. Second-order rate coefficients, at 25 and at 40°, of reaction (1) have been obtained for the substitution of tetramethyl-, tetra-n-propyl-, tetra-n-butyl-, tetraisobutyl-
-
Palladium-Mediated11C-Carbonylative Cross-Coupling of Alkyl/Aryl Iodides with Organostannanes: An Efficient Synthesis of Unsymmetrical Alkyl/Aryl [11C-carbonyl]Ketones作者:Farhad Karimi、Julien Barletta、Bengt LångströmDOI:10.1002/ejoc.200400883日期:2005.6Palladium-mediated 11C-carbonylative cross coupling of alkyl / aryl iodides with organostannanes. An efficient synthesis of un-symmetrical alkyl - aryl [carbonyl-11C]ketones
-
125Te-NMR-spektren von organotellurhalogeniden作者:H. Schumann、M. MagerstädtDOI:10.1016/s0022-328x(00)87641-x日期:1982.6Tetraorganotin compounds react with tellurium tetrachloride or tellurium tetrabromide with formation of the corresponding organotellurium trihalides and diorganotellurium dihalides. 125Te NMR spectra of the reaction mixtures are used to determine the reaction products.
-
Organometallic compounds 2. Mechanisms of electrophilic substitution of metal alkyls作者:M.H. Abraham、J.A. HillDOI:10.1016/s0022-328x(00)90821-0日期:1967.1The relative rates of electrophilic substitution of a number of series of metal alkysl of type RMXn(where X may = R), in which the alkyl group R varies along the series, have been interpreted in terms of the following mechanisms of substitution: (a) mechanism SE2, which results in a steric sequence of relative rate constants (R = Me > Et > Pr >iso-Pr >tert-Bu), (b) a newly-defined mechanisms SEC, which已经根据以下取代机理解释了RMX n类型(其中X可能= R)的多个系列金属烷基的亲电取代的相对速率,其中烷基R沿系列变化a)机制S E 2,这会产生相对速率常数的空间序列(R = Me> Et> Pr> iso-Pr> tert-Bu),(b)一种新定义的机制S E C,其结果是相对速率常数的极性序列(R = Me
-
X‐Ray Crystallography and Free Energy Calculations Reveal the Binding Mechanism of A <sub>2A</sub> Adenosine Receptor Antagonists作者:Willem Jespers、Grégory Verdon、Jhonny Azuaje、Maria Majellaro、Henrik Keränen、Xerardo García‐Mera、Miles Congreve、Francesca Deflorian、Chris Graaf、Andrei Zhukov、Andrew S. Doré、Jonathan S. Mason、Johan Åqvist、Robert M. Cooke、Eddy Sotelo、Hugo Gutiérrez‐de‐TeránDOI:10.1002/anie.202003788日期:2020.9.14was subsequently used to design new chromone derivatives. Their affinities for the A2AAR were experimentally determined and investigated through a cycle of ligand‐FEP calculations, validating the binding orientation of the different chemical substituents proposed. Subsequent X‐ray crystallography of the A2AAR with a low and a high affinity chromone derivative confirmed the predicted binding orientation我们提出了一个基于自由能微扰 (FEP) 计算、化学合成、生物物理绘图和 X 射线晶体学迭代的强大协议,以揭示拮抗剂系列与 A 2A腺苷受体 (AR)的结合模式。最初使用侧链 FEP 模拟对生物物理作图实验中的8 个 A 2A AR 结合位点突变进行分析,并在替代结合模式下进行。结果明显支持了一种结合模式,该模式随后被用于设计新的色酮衍生物。它们对 A 2A AR 的亲和力通过配体-FEP 计算循环进行实验测定和研究,验证了所提出的不同化学取代基的结合方向。随后对具有低亲和力和高亲和力色酮衍生物的 A 2A AR进行的 X 射线晶体学证实了预测的结合方向。这里报道的新分子和结构是由自由能计算驱动的,并提供了与 A 2A AR 结合的拮抗剂的新见解,A 2A AR 是免疫肿瘤学中的一个新兴靶点。
表征谱图
-
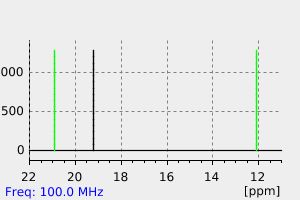
氢谱1HNMR
-
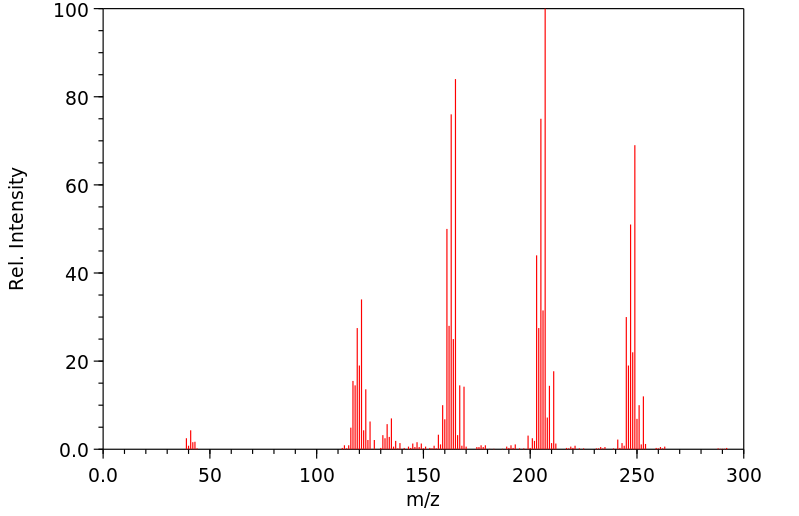
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锰,五羰基(三甲基甲锡烷基)-,(OC-6-22)-
锡烷,乙氧基三甲基-
锡烷,三丁基(1E)-1-庚烯基-
锡烷,三丁基(1-甲基-2-丁烯基)-,(Z)-
锡烷,三(1,1-二甲基乙基)乙炔基-
锡烷,(4-氯二环[2.2.1]庚-1-基)三甲基-
锡烷,(1E)-1-丁烯-3-炔基三丁基-
铝,三庚基-
铝,丁氧基二(2-甲基丙基)-
铅烷,三丁基-1-己炔基-
辛基锡
辛基氧代锡烷
膦,三(三甲基甲锡烷基)-
碳化铝
碘化三乙基铅
碘(三甲基)铅烷
硼烷胺,N,N-二(氯二甲基甲锡烷基)-1,1-二甲基-
硫烷负离子三甲基铅
硫代乙酸 S-[3-(三丁基锡烷基)丙基]酯
硒基二(三甲基锡)
癸酰(二羟基)铝
甲硫基三丁基锡烷
甲烷四基四(三甲基锡烷)
甲氧基二(2-甲基丙基)-铝
甲基锡
甲基烯丙基三正丁基锡
甲基氢化钼
甲基双(1-甲基环己基)锡烷
甲基二氯化铝
甲基三戊基锡
甲基(三丙基)锡烷
环己羧酸,2-氨基-,甲基酯,(1S,2S)-
环己基三异丙基锡烷
环己基[(三丁基锡烷基)氧基]重氮1-氧化物
环己基-三甲基锡烷
环己基(异丙基)二甲基锡烷
环丙基(三异丙基)锡烷
烯丙基三甲基锡烷
烯丙基三乙烯基锡烷
烯丙基三丁基锡
烯丙基三(3,3,4,4,5,5,6,6,7,7,8,8,8-十三氟辛基)锡烷
溴二乙基铝
溴三甲基铅
溴(异丙基)汞
溴(三乙基)铅
溴(三丁基)铅
氰酸三丁基锡烷
氯甲氧基甲基三丁基锡
氯甲基三甲基锡
氯化二己基铝