代谢
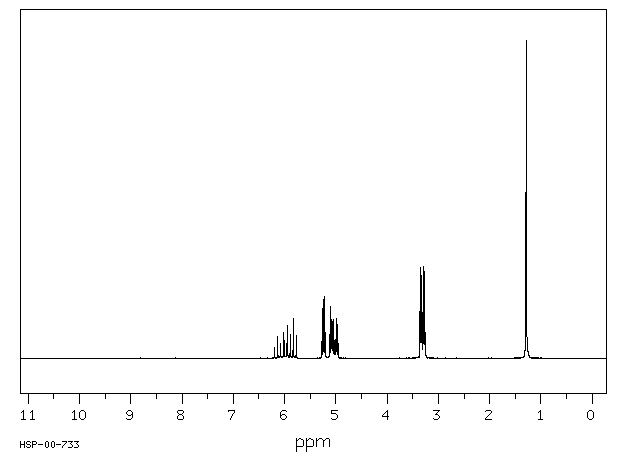
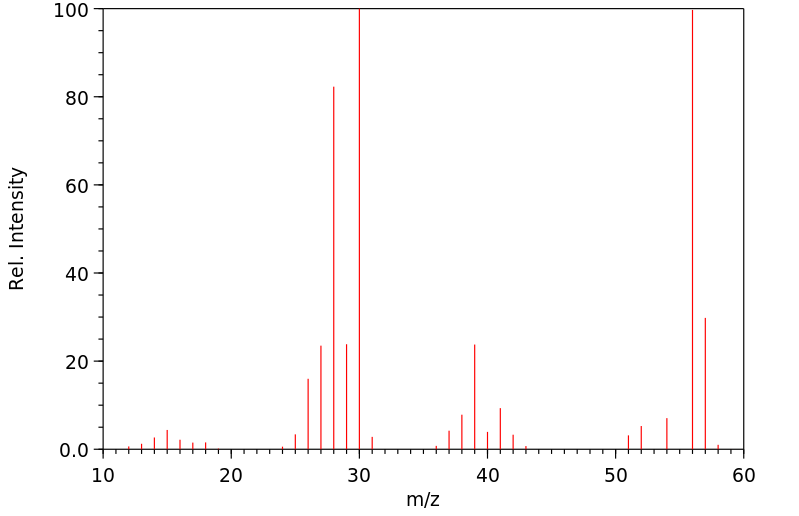
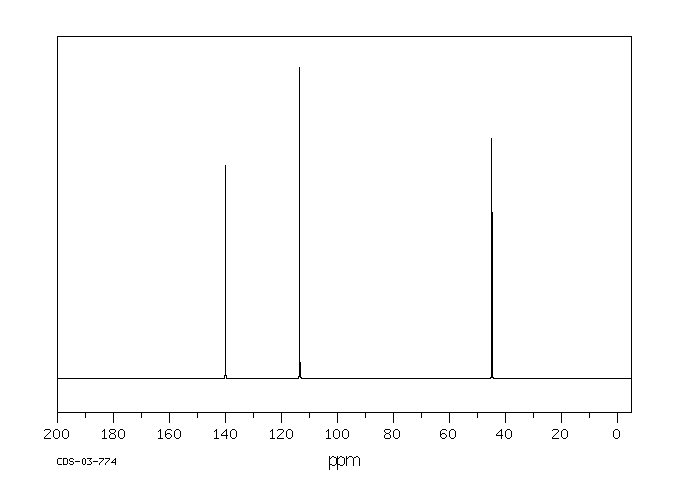
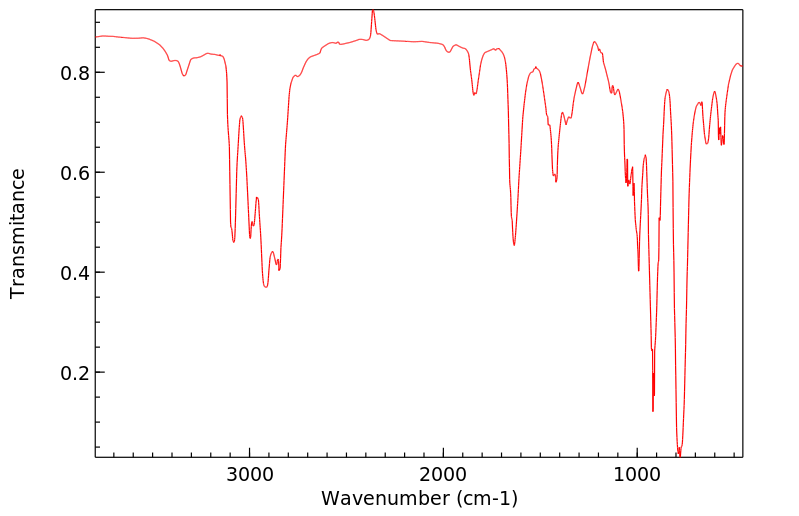
烯丙胺,一种已知的心血管毒素,在体外被代谢为丙烯醛,并且有人假设它作为一种远端毒素发挥作用。在这项研究中,通过质谱(MS)、核磁共振(NMR)和二维核磁共振光谱学鉴定并分离出了3-羟基丙基硫酸酯酸,它是烯丙胺在体内代谢的唯一尿液代谢物。平行实验显示,几个器官(在大动脉、血液和肺中最为显著)的还原型谷胱甘肽(GSH)水平降低,这与提出的丙烯醛中间体通过GSH结合途径的假设一致。这些发现表明,烯丙胺在体内被代谢为一种高度反应性的醛,然后通过GSH结合途径转化为硫酸酯酸。
... Allylamine, a known cardiovascular toxin, is metabolized in vitro to acrolein, /and/ has been hypothesized to act as a distal toxin. In this study, 3-hydroxypropylmercapturic acid was isolated and identified by MS, NMR, and 2D-NMR spectroscopy as the sole urinary metabolite of allylamine metabolism in vivo. Parallel experiments showed reduced glutathione (GSH) depletion in several organs (most marked in aorta, blood, and lung), which is consistent with GSH conjugation of the proposed acrolein intermediate. These findings indicate that allylamine was metabolized in vivo to a highly reactive aldehyde which was converted to a mercapturic acid through a GSH conjugation pathway. ...
来源:Hazardous Substances Data Bank (HSDB)