beta-氨基-1H-咪唑-5-丙醇盐酸盐
中文名称
beta-氨基-1H-咪唑-5-丙醇盐酸盐
中文别名
——
英文名称
2-amino-3-(1H-imidazol-5-yl)propan-1-ol;hydron;dichloride
英文别名
——
CAS
——
化学式
C6H13Cl2N3O
mdl
——
分子量
214.09
InChiKey
FRCAFNBBXRWXQA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
反应信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-6.5
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:74.9
-
氢给体数:5
-
氢受体数:5
文献信息
-
N-SUBSTITUTED PYRROLIDINES申请人:Chu Xin-Jie公开号:US20120010235A1公开(公告)日:2012-01-12Compounds of formula and enantiomers and pharmaceutically acceptable salts thereof are described, as well as the pharmaceutical compositions containing said compounds and their pharmaceutically acceptable salts, and the use of said compounds and pharmaceutical compositions for the treatment, control, amelioration or prevention of cancer.描述了公式的化合物及其对映体和药用可接受的盐,以及含有该化合物及其药用可接受的盐的药物组合物,以及该化合物和药物组合物用于治疗、控制、改善或预防癌症的用途。
-
Histidinol dehydrogenase protein, DNA and muteins and transgenic plants thereof申请人:CIBA-GEIGY AG公开号:EP0478502A2公开(公告)日:1992-04-01The present invention comprises cDNA coding for histidinol dehydrogenase from plants, the final step in histidine biosynthesis. The invention also comprises a novel method of purifying histidinol dehydrogenase from plants to essential honogeneity, the purified histidinol dehydrogenase, an assay for identifying inhibitors of histidinol dehydrogenase, an assay to identify mutants of histidinol dehydrogenase that are not inhibited by inhibitors of wild-type histidinol dehydrogenase, the inhibitors so identified as well as herbicide compositions containing them, the non-inhibited mutants of histidinol dehydrogenase, transgenic crop plants containing the non-inhibited mutants of histidinol dehydrogenase, and methods of treating weeds utilizing the application of histidinol dehydrogenase inhibitors to the transgenic crops containing the non-inhibited mutants of histidinol dehydrogenase.
-
Urea nucleosides as therapeutic and diagnostic agents申请人:GILEAD SCIENCES, INC.公开号:US20030069414A1公开(公告)日:2003-04-10Modified nucleosides and methods of making and using the nucleosides are disclosed. The compounds can be prepared by reacting nucleoside starting materials that contain a suitable leaving group at one or more of the carbon atoms in the purine or pyrimidine ring, with a vinylstannane, carbon monoxide, and a palladium catalyst to provide 1-ene-3-one intermediates. These intermediates are then reacted with suitably functionalized primary or secondary amines via a Michael reaction. When the intermediate is a 5-position modified pyrimidine ring, and the amine contains a second hydrogen, it can do a second Michael reaction with the ene-one or the ene-imine in the pyrimidine ring. Appropriate modification of the amine reactant can yield products with various bioactivities. The nucleosides can be used therapeutically as anti-cancer, anti-bacterial or anti-viral drugs. The nucleosides can also be used for diagnostic applications, for example, by incorporating a radiolabel or fluorescent label into the molecule. The nucleosides can be used to prepare oligonucleotides for use in various applications, either alone or in combination with other modified nucleosides and/or naturally occurring nucleosides.本发明公开了修饰核苷以及制造和使用核苷的方法。这些化合物的制备方法是将在嘌呤或嘧啶环的一个或多个碳原子上含有合适离去基团的核苷起始原料与乙烯基锡烷、一氧化碳和钯催化剂反应,生成 1-烯-3-酮中间体。然后,这些中间体通过迈克尔反应与适当官能化的伯胺或仲胺发生反应。当中间体是一个 5 位修饰的嘧啶环,并且胺中含有第二个氢时,它可以与嘧啶环中的烯酮或烯亚胺发生第二次迈克尔反应。对胺反应物进行适当的修饰,可以得到具有各种生物活性的产物。核苷可用作抗癌、抗菌或抗病毒药物。核苷还可用于诊断,例如在分子中加入放射性标记或荧光标记。核苷可用于制备寡核苷酸,单独使用或与其他改性核苷和/或天然核苷结合使用,以用于各种应用。
-
Reagent for the isolation of RNA申请人:Invitrogen Corporation公开号:US20030078412A1公开(公告)日:2003-04-24The present invention provides RNA extraction reagents, methods and kits that are especially useful for extracting RNA. The reagents, methods and kits of the present invention are especially useful for extracting RNA, for example, cytoplasmic RNA, from difficult materials, from plants, especially, difficult plant tissues, such as those containing phenolics, tannins, polysaccharides (such as starch) and resins. Comparative high yields are obtainable according to the present invention when compared to conventional reagents and methods. The RNA preparations obtained in accordance with the present invention are also of high quality as demonstrated by superior A 260/280 results and by gel electrophoresis.
-
RNA isolation reagent and methods申请人:Invitrogen Corporation公开号:US20030204077A1公开(公告)日:2003-10-30The invention provides methods, extraction reagents and kits for RNA isolation from eukaryotic cells such as plant or animal cells, where a phenol, a phenol solubilizer, a chelator and a nonionic detergent are used in the extraction reagent to replace the need for chaotropes and/or RNase inhibitors.
表征谱图
-
氢谱1HNMR
-
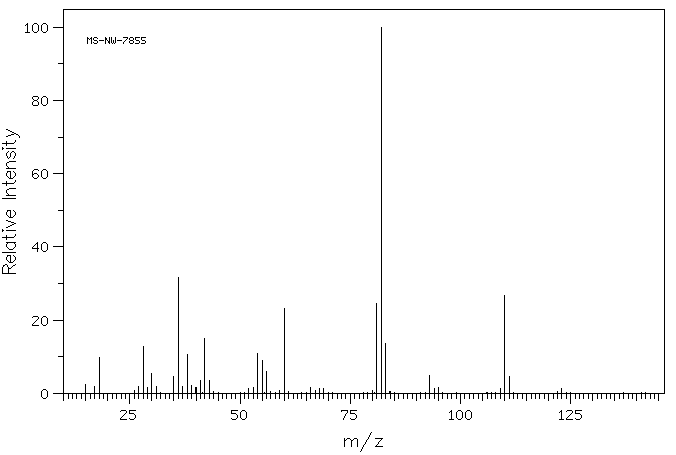
质谱MS
-
碳谱13CNMR
-
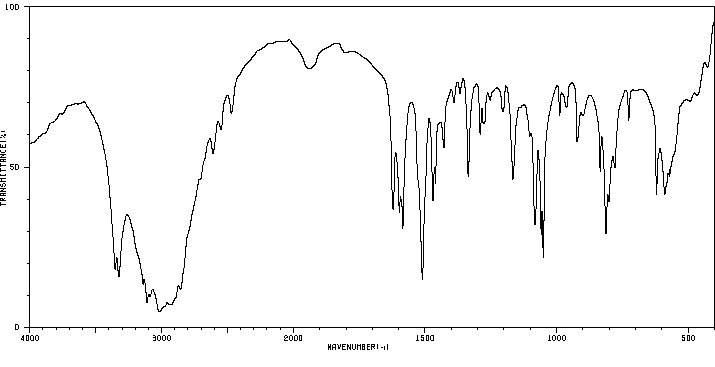
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷