丙炔醛 | 624-67-9
中文名称
丙炔醛
中文别名
炔丙醛
英文名称
Propargylic aldehyde
英文别名
propargyl aldehyde;propynal;2-Propynal;prop-2-ynal
CAS
624-67-9
化学式
C3H2O
mdl
——
分子量
54.0483
InChiKey
IJNJLGFTSIAHEA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:121-122 °C
-
沸点:58.85°C
-
密度:0.9152
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)、甲醇
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险类别码:R10
-
海关编码:2912190090
-
危险品运输编号:UN 1989
SDS
反应信息
-
作为反应物:参考文献:名称:Huettel, Chemische Berichte, 1941, vol. 74, p. 1828,1829摘要:DOI:
-
作为产物:描述:参考文献:名称:具有异噻唑并乙烯基侧链的新型1β-甲基卡宾烯的合成和生物学评估。摘要:描述了在吡咯烷环的C-5位置带有异噻唑并乙烯基的新型1β-甲基碳烯化合物1a,b的合成及其生物学评价。两种化合物均显示出有效且均衡的抗菌活性以及对DHP-1的高稳定性。特别是,与5-异恶唑衍生物2,亚胺培南和美洛培南相比,5-异噻唑衍生物1a表现出出色的DHP-1稳定性和先进的药代动力学特性。DOI:10.1016/s0960-894x(02)00948-4
-
作为试剂:描述:参考文献:名称:DE1001990摘要:公开号:
文献信息
-
Synthesis and spectroscopic properties of 1,4-diarylbutenynes作者:Alois H. A. Tinnemans、Wim H. LaarhovenDOI:10.1039/p29760001104日期:——Several new diarylbutenynes have been synthesized and analyses of the n.m.r. and u.v. spectra are described. For planar trans-isomers a conformational preference was found for 1-(α-naphthyl)- but not for 1-(β-naphthyl)-4-arylbutenynes. This difference is also found between compounds with C-1 attached to the α- or β-position of a larger aryl group.
-
Mono- and Bimetallic Aluminum Alkyl, Alkoxide, Halide and Hydride Complexes of a Bulky Conjugated Bis-Guanidinate(CBG) Ligand and Aluminum Alkyls as Precatalysts for Carbonyl Hydroboration作者:Thota Peddarao、Nabin Sarkar、Sharanappa NembennaDOI:10.1021/acs.inorgchem.9b03778日期:2020.4.6easily deprotonated (at least two protons) upon treatment with metal reagents. Both mono- and dinuclear aluminum alkyls and mononuclear aluminum alkoxide, halide, and hydride complexes have been structurally characterized. Further, we have demonstrated the potential of mononuclear, six-membered CBG aluminum dialkyls in catalytic hydroboration of a broad range of aldehydes and ketones with pinacolborane (HBpin)四芳基取代的对称共轭双胍(CBG)配体,例如L 1–3(3H)[L(3H)= (ArHN)(ArHN)C═N–C═NAr(NHAr)};Ar = 2,6-Me 2 -C 6 H 3(L 1(3H)),2,6-Et 2 -C 6 H 3(L 2(3H))和2,6- i Pr 2 -C 6 ħ 3(L 3(3H))]已被用于合成一系列的四和六元杂环铝(1 - 8),用于在第一时间。通常,带有N,N的铝配合物che-螯合的胍盐和β-二酮/二吡咯亚甲基配体系统分别形成四元和六元杂环。然而,共轭双胍配体具有在同一分子内形成具有多金属中心的四元和六元杂环的能力。这是由于存在三个酸性质子,它们在用金属试剂处理后很容易被去质子化(至少两个质子)。单核和双核烷基铝和单核铝醇盐,卤化物和氢化物络合物均已进行结构表征。此外,我们已经证明了单核六元CBG铝二烷基酯在与频哪醇硼烷(HBpin)催化的广泛醛和酮的硼氢化反应中的潜力。
-
Biomimetic Synthesis of Lankacidin Antibiotics作者:Kuan Zheng、Defeng Shen、Ran HongDOI:10.1021/jacs.7b08500日期:2017.9.20We devised short syntheses of lankacidinol and lankacyclinol that feature biomimetic Mannich macrocyclization. The modular construction of the carbon framework of these compounds is amenable to rapid structural diversification for the development of antibiotic and antitumor agents.我们设计了具有仿生曼尼希大环化特征的兰卡西林醇和兰卡环素的短合成。这些化合物的碳框架的模块化结构适合于开发抗生素和抗肿瘤剂的快速结构多样化。
-
Collective synthesis of natural products by means of organocascade catalysis作者:Spencer B. Jones、Bryon Simmons、Anthony Mastracchio、David W. C. MacMillanDOI:10.1038/nature10232日期:2011.7Organic chemists are now able to synthesize small quantities of almost any known natural product, given sufficient time, resources and effort. However, translation of the academic successes in total synthesis to the large-scale construction of complex natural products and the development of large collections of biologically relevant molecules present significant challenges to synthetic chemists. Here we show that the application of two nature-inspired techniques, namely organocascade catalysis and collective natural product synthesis, can facilitate the preparation of useful quantities of a range of structurally diverse natural products from a common molecular scaffold. The power of this concept has been demonstrated through the expedient, asymmetric total syntheses of six well-known alkaloid natural products: strychnine, aspidospermidine, vincadifformine, akuammicine, kopsanone and kopsinine. By combining two biosynthetic principles that have evolved in the natural world, David MacMillan and colleagues at the Merck Center for Catalysis at Princeton University, New Jersey, have developed a powerful strategy for the production of a broad spectrum of natural products. The first technique is organocascade catalysis, in which a continuous catalytic cascade replaces the traditional stop-go method of synthesis. The second is collective synthesis, in which a general synthetic route is used to reach a common molecular scaffold that, with appropriate fine-tuning, serves as a conduit to other members of the same chemical family. The method is demonstrated with the asymmetric total syntheses of six high-profile alkaloids: strychnine, aspidospermidine, vincadifformine, akuammicine, kopsanone and kopsinine.有机化学家现在能够在足够时间、资源和努力下合成几乎所有已知的天然产物的小量样本。但是,将全合成的学术成功转化为复杂天然产物的大规模构建和相关生物分子的大量集合开发,对合成化学家来说是一个巨大的挑战。在这里,我们展示了两种自然启发的技术,即有机级联催化(organocascade catalysis)和集体天然产物合成(collective natural product synthesis),它们可以促进使用常见分子支架来制备一系列具有不同结构的天然产物有用量。这个概念的强大之处已通过六种著名的生物碱天然产物的方便的、不对称的全合成得到证明:马钱子碱、阿部碱、长春碱、奥斯卡宁、可乐定和可普森宁。通过结合自然界中已经进化出的两种生物合成原理,David MacMillan和他在新泽西州普林斯顿大学的默克催化中心的同事们开发了一种能够广泛生产天然产物的有力策略。第一种技术是有机级联催化,其中连续的催化级联取代传统的停-来方法合成。第二种是集体合成,其使用通用的合成路线来达到一个常见的分子支架,而这个支架通过适当的微调,成为了同一家族的其他成员的通道。这种方法通过六种著名生物碱的不对称全合成得到展示:马钱子碱、阿部碱、长春碱、奥斯卡宁、可乐定和可普森宁。
-
Iron(III) Chloride-Catalyzed Tandem Aza-Prins/Friedel-Crafts Cyclization of 2-Arylethyl-2,3-butadienyl Tosylamides and Aldehydes-An Efficient Synthesis of Benzo[<i>f</i>]isoquinolines作者:Weilong Lin、Jiajia Cheng、Shengming MaDOI:10.1002/adsc.201600107日期:2016.6.16An efficient approach for the synthesis of 1,2,3,4,5,6‐hexahydrobenzo[f]isoquinolines via a tandem aza‐Prins/Friedel–Crafts cyclization from 2‐arylethyl‐2,3‐butadienyl tosylamides and aldehydes has been developed. This iron(III) chloride‐catalyzed cascade cyclization at the room temperature with different types of aldehydes, such as aromatic and heteroaromatic aldehydes, alkyl aldehydes, and α,β‐unsaturated
表征谱图
-
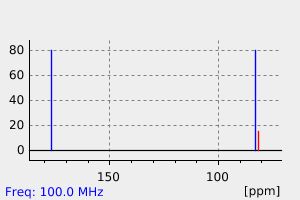
氢谱1HNMR
-
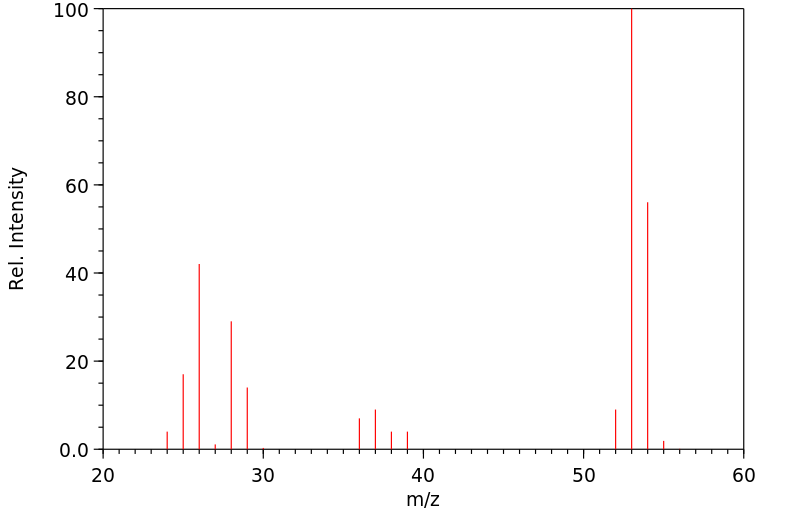
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锗烷,三甲基[3-(三甲基甲锡烷基)-2-炔丙基]-
锗烷,三甲基-2-炔丙基-
铜,1-戊炔基-
甲基炔丙基硫化物
甲基乙炔和丙二烯混合物
甲基丙-2-炔基氰基二硫代亚氨酸酯
甲基-D3-乙炔
环戊基乙炔
环己基乙炔
环丙乙炔
炔丙胺
炔丙基膦
炔丙基碘化物
炔丙基叔丁基二甲基硅烷
炔丙基三甲基硅烷
炔丙基三乙基硅烷
氘乙炔
戊-1-炔-3-胺
戊-1,3-二炔
戊-1,2-二烯-4-炔
异氰基-乙炔
己基(己-5-炔基)甲基硅烷
己-1-炔银
四碳化铀
反式-4-(2-丙炔基)-环己烷甲醇
双(三甲基锡)乙炔
双(三氟甲基)锌
十四碳-1,4-二炔
十四碳-1,3-二炔
十八碳-1,17-二炔
十八炔
十三碳-1,7-二炔
十三碳-1,12-二炔
十一碳-1,5-二炔
亚硫酸二(2-丙炔基)酯
二甲基炔丙基溴化硫
二炔丙基硫醚
二乙炔基-二甲基-锗烷
二丙-1-炔基汞
二[2-甲氧基乙基汞(II)]乙炔
二(三正丁基甲锡烷基)乙炔
二(3-羟基-1-丙炔基)汞(II)
乙炔锂乙二胺配合物
乙炔银
乙炔基环己烷钠
乙炔基环丙烷氯化镁
乙炔基(三甲基)锗烷
乙炔基(三甲基)硅烷铜(1+)
乙炔基(三甲基)硅烷溴化镁
乙炔基(三甲基)硅烷氯化镁