反式-3-乙氧基丙烯腈 | 58243-08-6
中文名称
反式-3-乙氧基丙烯腈
中文别名
——
英文名称
(E)-3-ethoxyacrylonitrile
英文别名
3-ethoxy-2-propenenitrile;(2E)-3-ethoxyacrylonitrile;3-Ethoxyacrylonitrile;β-ethoxy-acrylonitrile;3-ethoxypropenenitrile;(E)-3-ethoxyprop-2-enenitrile
CAS
58243-08-6
化学式
C5H7NO
mdl
——
分子量
97.1167
InChiKey
HUPVIAINOSTNBJ-HWKANZROSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:90-91 °C19 mm Hg(lit.)
-
密度:0.944 g/mL at 25 °C(lit.)
-
闪点:179 °F
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:33
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
SDS
| Name: | trans-3-Ethoxyacrylonitrile 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 58243-08-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 58243-08-6 | trans-3-Ethoxyacrylonitrile | 98% | 261-181-1 |
Risk Phrases: 10 22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Harmful if swallowed.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Will burn if involved in a fire. Flammable liquid and vapor.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Remove all sources of ignition.
Use a spark-proof tool.
Section 7 - HANDLING and STORAGE
Handling:
Use spark-proof tools and explosion proof equipment. Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Keep away from sources of ignition. Store in a cool, dry place.
Store in a tightly closed container. Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 58243-08-6: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 50 deg C ( 122.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H7NO
Molecular Weight: 97.12
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources.
Incompatibilities with Other Materials:
Strong oxidizing agents, bases.
Hazardous Decomposition Products:
Hydrogen cyanide, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 58243-08-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
trans-3-Ethoxyacrylonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 10 Flammable.
R 22 Harmful if swallowed.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
WGK (Water Danger/Protection)
CAS# 58243-08-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 58243-08-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 58243-08-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Process for the preparation of cytosines摘要:可以选择在5位位置进行替代的胞嘧啶经过一种简化的过程制备,其中尿素与醇盐和烷氧基丙烯腈或二烷氧基丙腈在一种稀水溶性有机溶剂中反应,该溶剂在反应条件下是惰性的。使用这样的溶剂使得可以简单地反应所述的反应物,而无需分离中间阶段,并且可以在不经过单独纯化的情况下重复使用有机溶剂。公开号:US05026852A1
-
作为产物:描述:参考文献:名称:The Synthesis and Reactions of β-Chloroacrylonitrile摘要:DOI:10.1021/jo01030a033
文献信息
-
N-[2-(pyridinyl)-4-pyrimidinyl]ureas申请人:Sterling Drug Inc.公开号:US04008235A1公开(公告)日:1977-02-15Compounds useful as anti-allergic agents are N-R.sub.3 -N-R.sub.4 -N'-R-N'-(2-Q-5-R.sub.1 -6-R.sub.2 -4-pyrimidinyl)ureas (I), where Q is 4- or 3- or 2-pyridinyl or 4- or 3- or 2-pyridinyl having one or two lower-alkyl substituents or N-oxide thereof, R is hydrogen or lower-alkyl, R.sub.1 is hydrogen, lower-alkyl or cyano, R.sub.2 is hydrogen or lower-alkyl, R.sub.3 is hydrogen, lower-alkyl or lower-hydroxyalkyl, R.sub.4 is hydrogen, lower-alkyl, lower-hydroxyalkyl, lower-alkenyl or lower-cycloalkyl. Said ureas are prepared by reacting 2-Q-4-RNH-5-R.sub.1 -6-R.sub.2 -pyrimidine (II) with a carbamylating agent selected from an R.sub.4 '-isocyanate of the formula R.sub.4 'N=C=O to produce N-R.sub.4 '-N'-R-N'-(2-Q-5-R.sub.1 -6-R.sub.2 -4-pyrimidinyl)urea (IA), an N-R.sub.3 '-N-R.sub.4 '-carbamyl halide of the formula R.sub.3 'R.sub.4 'NC(=O)-halide to produce N-R.sub.3 '-N-R.sub.4 'N'-R-N'-(2-Q-5-R.sub.1 -6-R.sub.2 -4-pyrimidinyl)urea (IB) or 1,1'-carbonyldiimidazole to produce N-(2-Q-5-R.sub.1 -6-R.sub.2 -4-pyrimidinyl)-N-R-imidazole-1-carboxamide and then reacting said 1-carboxamide with R.sub.3 R.sub.4 NH to produce I.可用作抗过敏剂的化合物为N-R.sub.3 -N-R.sub.4 -N'-R-N'-(2-Q-5-R.sub.1 -6-R.sub.2 -4-嘧啶基)脲类化合物(I),其中Q为4-或3-或2-吡啶基或带有一个或两个较低烷基取代基或其N-氧化物,R为氢或较低烷基,R.sub.1为氢、较低烷基或氰基,R.sub.2为氢或较低烷基,R.sub.3为氢、较低烷基或较低羟基烷基,R.sub.4为氢、较低烷基、较低羟基烷基、较低烯基或较低环烷基。所述脲类化合物通过将2-Q-4-RNH-5-R.sub.1 -6-R.sub.2 -嘧啶(II)与选择自R.sub.4 'N=C=O的R.sub.4 '-异氰酸酯的羰基化试剂反应制备,以产生N-R.sub.4 '-N'-R-N'-(2-Q-5-R.sub.1 -6-R.sub.2 -4-嘧啶基)脲(IA),R.sub.3 'R.sub.4 'NC(=O)-卤化物的N-R.sub.3 '-N-R.sub.4 '-脲基卤化物以产生N-R.sub.3 '-N-R.sub.4 'N'-R-N'-(2-Q-5-R.sub.1 -6-R.sub.2 -4-嘧啶基)脲(IB)或1,1'-羰基二咪唑以产生N-(2-Q-5-R.sub.1 -6-R.sub.2 -4-嘧啶基)-N-R-咪唑-1-甲酰胺,然后将所述1-甲酰胺与R.sub.3 R.sub.4 NH反应以产生I。
-
2-Substituted-pyrido[2,3-d]pyrimidin-5(8H)-ones申请人:Sterling Drug Inc.公开号:US04560753A1公开(公告)日:1985-12-242-Q-6-Q'-8-R-pyrido[2,3-d]pyrimidin-5(8H)-ones (I), where Q is 4(or 3)-hydroxyphenyl, 4(or 3)-methoxyphenyl, 4(or 3)-pyridinyl or 4(or 3)-pyridinyl having one or two lower-alkyl substituents, R' is hydrogen or alkyl having one to four carbon atoms, Q is hydrogen, amino or nitro, and R is alkyl having from one to four carbon atoms, CH(C.sub.2 H.sub.5).sub.2, (CH.sub.2).sub.n .dbd.CHCH.sub.2 where n is 1 or 2, or Y--Z where Y is alkylene having from two to four carbon atoms and having its connecting linkages on different carbon atoms and Z is hydroxy, OR.sub.1 or NR.sub.1 R.sub.2 where R.sub.1 and R.sub.2 are each methyl or ethyl, or acid-addition salts thereof, and their preparation are shown. Also shown is the cardiotonic use of I where Q is 4(or 3)-hydroxyphenyl, 4(or 3)-pyridinyl or 4(or 3)-pyridinyl having one or two lower-alkyl substituents and Q' is hydrogen or amino.2-Q-6-Q'-8-R-吡啶并[2,3-d]嘧啶-5(8H)-酮(I),其中Q为4(或3)-羟基苯基、4(或3)-甲氧基苯基、4(或3)-吡啶基或带有一个或两个较低烷基取代基的4(或3)-吡啶基,R'为氢或具有一至四个碳原子的烷基,Q为氢、氨基或硝基,R为具有一至四个碳原子的烷基,CH(C.sub.2 H.sub.5).sub.2、(CH.sub.2).sub.n .dbd.CHCH.sub.2,其中n为1或2,或Y--Z,其中Y为具有两至四个碳原子的烷基,并且其连接键连接在不同的碳原子上,Z为羟基、OR.sub.1或NR.sub.1 R.sub.2,其中R.sub.1和R.sub.2各自为甲基或乙基,或其酸盐加合物,以及它们的制备方法。还显示了I的强心用途,其中Q为4(或3)-羟基苯基、4(或3)-吡啶基或带有一个或两个较低烷基取代基的4(或3)-吡啶基,Q'为氢或氨基。
-
2-(Pyridinyl or hydroxyphenyl)-8-substituted申请人:Sterling Drug Inc.公开号:US04432981A1公开(公告)日:1984-02-212-Q-6-Q'-8-R-pyrido[2,3-d]pyrimidin-5(8H)-ones (I), where Q is 4(or 3)-hydroxyphenyl, 4(or 3)-methoxyphenyl, 4(or 3)-pyridinyl or 4(or 3)-pyridinyl having one or two lower-alkyl substituents, R' is hydrogen or alkyl having one to four carbon atoms, Q is hydrogen, amino or nitro, and R is alkyl having from one to four carbon atoms, CH(C.sub.2 H.sub.5).sub.2, (CH.sub.2).sub.n .dbd.CHCH.sub.2 where n is 1 or 2, or Y-Z where Y is alkylene having from two to four carbon atoms and having its connecting linkages on different carbon atoms and Z is hydroxy, OR.sub.1 or NR.sub.1 R.sub.2 where R.sub.1 and R.sub.2 are each methyl or ethyl, or acid-addition salts thereof, and their preparation are shown. Also shown is the cardiotonic use of I where Q is 4(or 3)-hydroxyphenyl, 4(or 3)-pyridinyl or 4(or 3)-pyridinyl having one or two lower-alkyl substituents and Q' is hydrogen or amino.2-Q-6-Q'-8-R-吡啶并[2,3-d]嘧啶-5(8H)-酮(I),其中Q为4(或3)-羟基苯基,4(或3)-甲氧基苯基,4(或3)-吡啶基或带有一个或两个较低烷基取代基的4(或3)-吡啶基,R'为氢或具有一至四个碳原子的烷基,Q为氢,氨基或硝基,R为具有一至四个碳原子的烷基,CH(C.sub.2 H.sub.5).sub.2,(CH.sub.2).sub.n .dbd.CHCH.sub.2其中n为1或2,或Y-Z其中Y为具有两至四个碳原子的烷基,并且其连接键连接在不同的碳原子上,Z为羟基,OR.sub.1或NR.sub.1 R.sub.2其中R.sub.1和R.sub.2各自为甲基或乙基,或其酸盐加合物,以及它们的制备方法。还显示了I的强心用途,其中Q为4(或3)-羟基苯基,4(或3)-吡啶基或带有一个或两个较低烷基取代基的4(或3)-吡啶基,Q'为氢或氨基。
-
Insecticidal 5-substituted-2,4-diaminopyrimidine derivatives申请人:FMC Corporation公开号:US05521192A1公开(公告)日:1996-05-28An insecticidal composition comprising, in admixture with an agriculturally acceptable carrier, an insecticidally effective amount of a compound of the formula: ##STR1## wherein R, R.sup.1, R.sup.2, R.sup.3, R.sup.7, R.sup.8, m, n, and p are as defined herein, and agriculturally acceptable salts thereof, and methods of using the same.一种杀虫组合物,与农业可接受的载体混合,包括化学式为:##STR1## 的化合物的杀虫有效量,其中R、R.sup.1、R.sup.2、R.sup.3、R.sup.7、R.sup.8、m、n和p如本文所定义,以及其农业可接受的盐,以及使用方法。
-
[EN] 7-PHENOXYCHROMAN CARBOXYLIC ACID DERIVATIVES<br/>[FR] DÉRIVÉS D'ACIDE 7-PHÉNOXYCHROMANECARBOXYLIQUE申请人:ARRAY BIOPHARMA INC公开号:WO2010075200A1公开(公告)日:2010-07-01Compounds of Formula I: (I) in which A, A1, R1, R7a, R7b, R8 and R10 have the meanings given in the specification, are DP2 receptor inhibitors useful in the treatment of useful in the treatment and prevention of immunologic diseases, allergic diseases such as asthma, allergic rhinitis and atopic dermatitis, and other inflammatory diseases mediated by prostaglandin D2 (PGD2). The compounds of Formula I may also be useful in treating diseases or medical conditions involving the Th2 T cell via production of IL-4, IL-5 and/or IL-13.
表征谱图
-
氢谱1HNMR
-
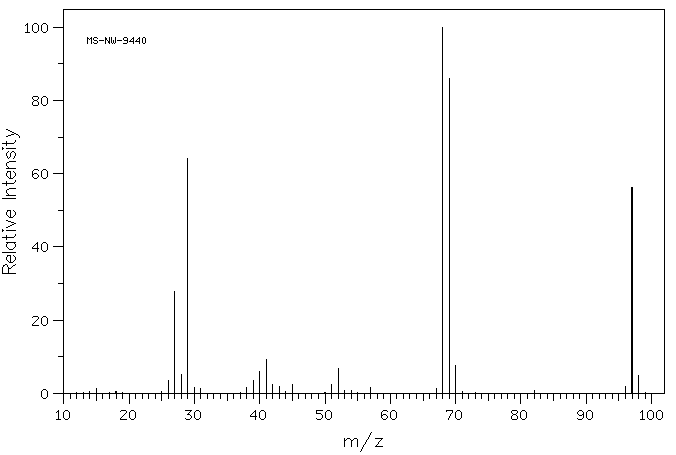
质谱MS
-
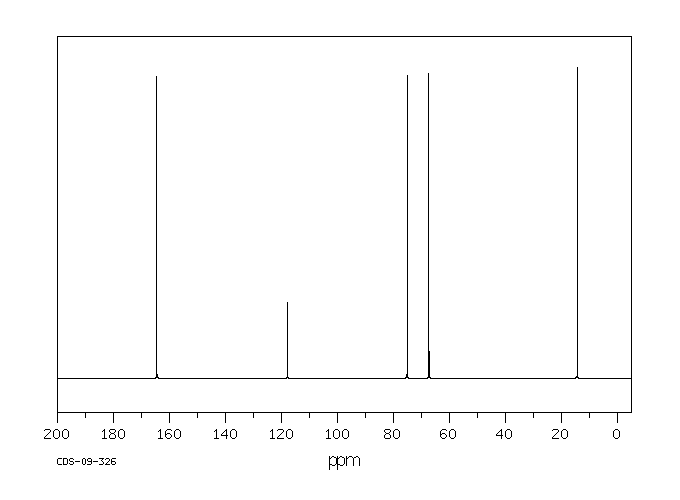
碳谱13CNMR
-
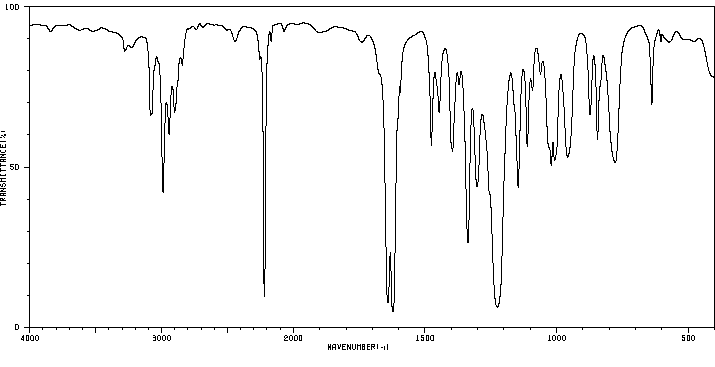
红外IR
-
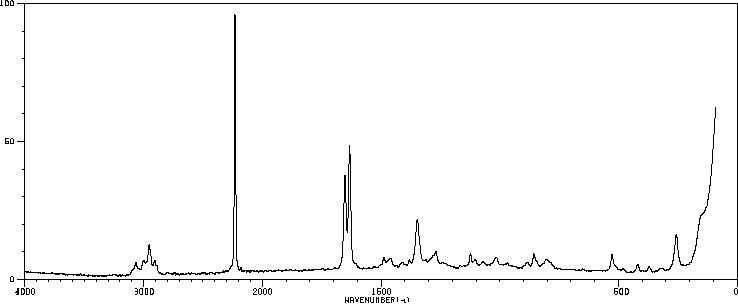
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷